Product Overview
Testosterone cream is a pharmacist-compounded, semisolid preparation designed to deliver the principal endogenous androgen, testosterone, across intact skin, thereby restoring physiologic serum concentrations in individuals with clinically confirmed androgen deficiency. Unlike parenteral esters that generate supraphysiologic peaks and subsequent troughs, or oral alkylated formulations that undergo extensive first-pass hepatic metabolism, properly formulated topical systems yield a gradual, sustained absorption profile, mitigating volatility in circulating hormone levels while avoiding hepatic exposure.[1]
Historically, transdermal delivery emerged in response to limited patient acceptance of intramuscular injections and median seven-day patch adhesion failures. Early hydro-alcoholic gels revolutionized home administration but carried drawbacks, notably inadvertent transfer to close contacts and irritation from volatile solvents. Consequently, compounding pharmacists refined cream bases with optimized lipid partition coefficients and penetration enhancers such as oleic acid and isopropyl myristate, permitting individualization of dose strengths from 1 mg/mL up to 250 mg/mL within either 15 mL or 30 mL pump dispensers tailored to patient need.[2]
The versatility of weight-based titration-dispensing one metered pump actuation equating to a known milligram output-allows clinicians to escalate therapy by as little as 1 mg/mL weekly, minimizing erythrocytosis risk while achieving target troughs between 400 ng/dL and 700 ng/dL. Cream vehicles further permit site rotation to reduce folliculitis and accommodate patient preference for non-greasy, quick-drying textures.[3]
Compounded testosterone cream falls under Section 503A of the Federal Food, Drug, and Cosmetic Act, meaning it is prepared pursuant to an individually written prescription for a specific patient in a state-licensed pharmacy, using United States Pharmacopeia grade active pharmaceutical ingredient dissolved into a validated anhydrous base with demonstrated beyond-use dates supported by stability-indicating assays.[4]
Because the formulation is not FDA-approved, prescribers must discuss the investigational nature of compounded therapy, emphasize mandatory baseline and ongoing laboratory surveillance (hemoglobin/hematocrit, prostate-specific antigen, liver enzymes, lipid profile), and ensure patients understand that consistent application technique, skin preparation, and adherence to wash-off precautions are critical to therapeutic success and safety.[5]
Initial dosing commonly begins at 10 mg total daily testosterone applied to hairless skin areas; however, compounded preparations permit micro-adjustment. Providers may prescribe 1 mg/mL concentration and instruct one pump actuation delivering 0.5 mL (0.5 mg) twice daily, titrating upward in weekly 1 mg/mL increments based on trough serum testosterone drawn 12 hours post-application, hemoglobin levels, and symptom response.[21]
Higher concentrations (e.g., 100 mg/mL) afford smaller application volumes for patients requiring substantial replacement yet wishing to minimize skin surface coverage; nevertheless, increasing concentration can augment DHT conversion locally, so split-dose regimens or alternate anatomical sites may balance efficacy with androgenic cutaneous effects.[22]
Endogenous testosterone exerts biological activity after diffusion into target cells, 5α-reduction to dihydrotestosterone (DHT) in tissues such as skin and prostate, and binding to the cytosolic androgen receptor (AR). The ligand-receptor complex translocates to the nucleus, where it dimerizes, associates with androgen-response elements, and modulates transcription of genes governing virilization, erythropoiesis, bone density, and mood.[6]
Transdermal creams leverage the skin’s stratum corneum as both barrier and depot, exploiting a concentration gradient that drives testosterone into the viable epidermis and dermal microcirculation. Penetration enhancers disrupt lipid bilayer packing or increase solvent thermodynamic activity, while occlusion by the cream base maintains hydration, further facilitating permeation.[7]
Once in systemic circulation, testosterone is bound to sex hormone-binding globulin (SHBG) and albumin; only the free fraction is biologically active. Topical delivery avoids hepatic first-pass metabolism that converts testosterone to inactive epitestosterone and androstenedione, thereby achieving a higher bioavailability per milligram applied compared with oral or buccal routes.[8]
Pharmacokinetic studies reveal that scrotal or inner-thigh application sites demonstrate approximately fourfold higher flux than shoulder or upper-arm skin because regional differences in stratum corneum thickness and 5α-reductase activity augment local conversion to DHT, which itself possesses greater AR affinity.[9]
Testosterone cream is contraindicated in individuals with known hypersensitivity to testosterone or any excipients in the base vehicle, including penetration enhancers or preservatives that may precipitate allergic or irritant contact dermatitis.[10]
Men with carcinoma of the breast or known or suspected prostate cancer must not receive exogenous androgens, as stimulation of androgen-responsive neoplastic tissue could accelerate tumor progression; therefore, screening digital rectal examination and age-appropriate PSA testing remain obligatory prior to initiation and during therapy.[11]
Serious caution applies to patients with uncontrolled heart failure, untreated severe obstructive sleep apnea, polycythemia (hematocrit > 54 %), or poorly controlled hypertension, since testosterone-mediated erythropoiesis, fluid retention, and alterations in vascular tone could exacerbate these conditions, necessitating either stabilization of comorbidities or selection of alternative modalities before considering topical therapy.[12]
Testosterone is primarily metabolized by hepatic CYP3A4; potent inhibitors (e.g., azole antifungals, ritonavir) may raise serum testosterone and DHT concentrations, whereas inducers (e.g., carbamazepine, rifampin) can reduce efficacy.[13]
Clinically significant potentiation of the anticoagulant effect of warfarin has been documented, attributed to multidimensional changes in clotting factor synthesis and metabolism. INR should be monitored closely following androgen initiation or dose adjustments, and warfarin requirements may decline.[14]
Corticosteroids, growth hormone, and insulin sensitizers may interact with testosterone’s anabolic effects on protein metabolism, potentially necessitating dosage adjustments of these agents to avoid additive edema or alterations in glucose homeostasis; prescribers should evaluate cumulative endocrine load in polypharmacy scenarios.[15]
Local adverse events include erythema, pruritus, folliculitis, and in rare cases, ulcerative irritant dermatitis at application sites owing to penetration enhancer or occlusion effects; prudent site rotation and barrier repair emollients mitigate incidence.[16]
Systemic events mirror those of other androgen formulations: erythrocytosis leading to hyper viscosity; acneiform eruptions from heightened sebaceous activity; and, in predisposed individuals, acceleration of androgenic alopecia. Periodic complete blood counts and dermatologic evaluations are prudent.[17]
Cardiometabolic signals warrant vigilance: supraphysiologic dosing can unfavorably alter lipid panels, elevate blood pressure via sodium retention, and, although recent randomized data suggest cardiovascular non-inferiority in properly selected men, longitudinal registries continue to explore associations with myocardial infarction and stroke, underscoring the need for shared decision-making and individualized risk assessment.[18]
Exposure to testosterone during the first trimester carries the theoretical risk of virilizing a female fetus, as the masculinization programming window spans critical organogenesis weeks; thus, the product is contraindicated in pregnancy and labelled Category X. Partners of users should avoid direct skin contact with unwashed application sites, and male patients should wash hands immediately after dosing to prevent secondary exposure.[19]
Limited lactation data exist; however, given testosterone’s lipophilicity and potential to suppress prolactin, compounded cream should be avoided in breastfeeding individuals unless benefits outweigh risks and safer alternatives are unsuitable, with the caveat that systemic absorption by the nursing infant has not been adequately quantified.[20]
Compounded testosterone cream should be stored at 20 °C to 25 °C (controlled room temperature) with permissible excursions between 15 °C and 30 °C, protected from direct sunlight and excessive moisture to preserve emulsion integrity and prevent oxidative degradation of the steroid nucleus.[23]
Validated beyond-use date studies employing high-performance liquid chromatography indicate chemical potency and microbiological stability for up to 180 days when manufactured under USP <795> non-sterile conditions in an anhydrous base using nitrogen blanket filling and light-resistant packaging; nevertheless, pharmacists must label each lot with the shorter of 90 days or manufacturer-supported dating when antioxidants are omitted.[24]
- Bhasin, S., Brito, J. P., Cunningham, G. R., et al. (2018). Testosterone therapy in men with hypogonadism: An Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. https://endocrine.org/clinical-practice-guidelines/testosterone-therapy
- Cloud, J. (2014, Aug 18). Manopause?! Aging, insecurity and the $2 billion testosterone industry. TIME. https://time.com/3062889/manopause-aging-insecurity-and-the-2-billion-testosterone-industry/
- Olsson, J., et al. (2014). Pharmacokinetics and bioavailability of a new 2% testosterone gel. Endocrine. https://medirequests.com/pdfs/Olsson%20et%20al%202014t.pdf
- Liu, A., et al. (2017). Pharmacokinetics of testosterone cream applied to scrotal skin. Andrology, 5(5), 776-784. https://doi.org/10.1111/andr.12357
- Mulhall, J. P., et al. (2018). Evaluation and management of testosterone deficiency: AUA guideline. https://www.auanet.org/documents/Guidelines/PDF/Testosterone-Deficiency-JU.pdf
- Heinlein, C. A., & Chang, C. (2002). Androgen receptor (AR) coregulators: An overview. Endocrine Reviews, 23(2), 175-200. https://doi.org/10.1210/edrv.23.2.0460
- Androgen Receptors (pp. 1-25). (2013). In Chang, C. (Ed.), Androgen Receptors: Mechanisms and Clinical Applications. Springer. https://link.springer.com/book/10.1007/978-1-4615-1161-8
- Swerdloff, R. S., et al. (2000). Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. The Journal of Clinical Endocrinology & Metabolism, 85(12), 4500-4510. https://doi.org/10.1210/jcem.85.12.7045
- Wang, C., et al. (2001). Pharmacokinetics of testosterone after percutaneous gel or buccal administration. Fertility and Sterility, 76(1), 32-40. https://doi.org/10.1016/S0015-0282(01)01827-1
- Anderson, E. J., et al. (2023). Cardiovascular safety of testosterone-replacement therapy. The New England Journal of Medicine, 389, 132-143. https://www.nejm.org/doi/pdf/10.1056/NEJMoa2215025
- Patel, A. S., et al. (2024). Association between testosterone therapy and cardiovascular disease: Systematic review and meta-analysis. Primary Care Update, 31, 45-57. https://doi.org/10.1016/j.pcu.2024.01.005
- Fernández-Balsells, M. M., et al. (2010). Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism, 95(6), 2560-2575. https://doi.org/10.1210/jc.2009-2575
- University of Indiana School of Medicine. (2025). Cytochrome P450 drug interaction table. https://drug-interactions.medicine.iu.edu/Main-Table.aspx
- Drugs..com. (2025). Testosterone and warfarin interactions. https://www.drugs.com/drug-interactions/testosterone-with-warfarin-2167-0-2311-0.html
- Barnes, T. J., & Yoon, E. Y. (2011). Drug interactions involving warfarin: Practice tool and practical management tips. Canadian Pharmacists Journal, 144(1), 21-28. https://cifav.it/res/download/pdf/152_it.pdf
- Basaria, S., et al. (2022). Adverse cardiovascular events and mortality during testosterone replacement: Lancet review. The Lancet Healthy Longevity, 3(12), e822-e834. https://www.thelancet.com/journals/lanhl/article/PIIS2666-7568(22)00096-4/fulltext
- .de Ronde, W., et al. (2009). Hyperandrogenism after transfer of topical testosterone gel: Case report. Human Reproduction, 24(2), 425-427. https://doi.org/10.1093/humrep/den379
- .De Santis, M., et al. (2011). Secondary exposure to testosterone from patients receiving replacement therapy. Current Medical Research and Opinion, 27(9), 1683-1692. https://doi.org/10.1185/03007995.2011.652255
- O’Shaughnessy, P. J., et al. (2025). Testosterone exposure during the fetal masculinization window. bioRxiv. https://www.biorxiv.org/content/10.1101/2025.02.11.637631v1
- Mahendroo, M., et al. (2019). Alternative androgen production pathways and masculinization. PLOS Biology, 17(11), e3000002. https://doi.org/10.1371/journal.pbio.3000002
- Kaminetsky, J., et al. (2020). Efficacy and safety of a new 2 % topical testosterone gel. Endocrine Practice, 26(9), 1108-1117. https://doi.org/10.4158/EP-2020-0161
- Dobs, A. S., et al. (2012). Pharmacokinetics and relative bioavailability of a 1.62 % testosterone gel. Endocrine Practice, 18(4), 550-559. https://doi.org/10.4158/EP11266.OR
- Medisca. (2024). Beyond-use date library: Stability studies. https://www.medisca.com/formulas/bud-library
- PCCA. (2016). Formulations subjected to beyond-use date testing: Testosterone 2 % cream. https://customcompounding.com.au/wp-content/uploads/2017/02/PCCA-BUD-TestostCrm_11850.pdf
- Verywell Health. (2024). Do men need hormone replacement therapy? https://www.verywellhealth.com/do-men-need-hormone-replacement-therapy-8639531
- O’Bryant, T., et al. (2023). Under-reporting supplements: Drug-induced liver injury due to testosterone booster. Military Medicine, 190(1-2), e453-e457. https://doi.org/10.1093/milmed/usad006
- Schulze, K., et al. (2015). Testosterone-receptor positive hepatocellular carcinoma in a young man. BMC Gastroenterology, 15, 64. https://doi.org/10.1186/s12876-015-0288-0
- Needham, S., & Needham, S. (2018). Absorption of testosterone cream via scrotal delivery: Case study. Alturas Analytics White Paper. https://excelmale.com/threads/case-study-absorption-of-testosterone-cream-via-scrotal-delivery.16797/
- TRTed Foundation. (2025). Should I worry about secondary transfer of my testosterone gel? https://www.trted.org/articles/should-i-be-worried-about-secondary-transfer-of-my-testosterone-gel
- Allan, C. A., et al. (2021). Safety, efficacy, and pharmacokinetics of oral testosterone undecanoate. Andrology, 9(4), 1212-1223. https://doi.org/10.1111/andr.13747
- T’Sjoen, G., et al. (2016). Testosterone therapy: Transdermal androgens. In Male Hypogonadism (pp. 221-240). Springer. https://link.springer.com/chapter/10.1007/978-3-319-46086-4_11
- Roberts, R. S., et al. (1998). Comparison of skin irritation potential of two testosterone transdermal systems. Journal of Dermatological Treatment, 9(1), 39-44. https://doi.org/10.3109/09546639809160500
- Dobs, A. S., et al. (1998). Topical corticosteroid pretreatment to reduce dermatitis from testosterone patch. Journal of Dermatological Treatment, 9(2), 111-116. https://doi.org/10.3109/09546639809160532
- Fischer, T., & Maibach, H. I. (1989). Contact dermatitis with transdermal therapeutic systems. Contact Dermatitis, 20(3), 189-195. https://doi.org/10.1111/j.1600-0536.1989.tb03087.x
- Sharma, V., et al. (2022). Transdermal patches: A new drug delivery approach. Medical Journal of Drug Development, 14(2), 123-130. https://mdpub.net/?mno=140224
How soon will symptoms improve after starting testosterone cream?
Many patients notice increased energy and libido within 3-4 weeks, though maximal benefits on muscle mass and bone density require 3-6 months of consistent use.[25]
Does topical testosterone raise the risk of heart attack?
Large, randomized trials show no statistically significant increase in major adverse cardiovascular events in appropriately screened men, but individual risk factors must be reassessed regularly.[26]
Can lifestyle changes replace testosterone therapy?
Weight loss, resistance training, and adequate sleep may elevate endogenous testosterone modestly, yet clinically meaningful deficiencies often require pharmacologic replacement for symptomatic relief.[27]
Is liver toxicity a concern with the cream?
Unlike some oral anabolic steroids linked to hepatic injury, transdermal formulations bypass first-pass metabolism and have not been associated with clinically significant hepatotoxicity in otherwise healthy livers.[28]
Will the cream worsen male-pattern baldness?
Genetic predisposition determines hair follicle sensitivity to DHT; topical testosterone may accelerate loss in susceptible individuals, so discussing prophylactic treatments is advisable.[29]
How can partners avoid accidental exposure?
Apply to covered areas such as upper thigh, allow complete drying, wash hands, and avoid skin-to-skin contact for at least two hours or after washing the site with soap and water.[30]
What if a dose is missed?
Apply it when remembered on the same day; if close to the next scheduled application, skip the missed dose-do not double to compensate, as this may provoke hormonal fluctuation.[31]
Are there natural alternatives?
Herbal “testosterone boosters” lack robust evidence and, in rare cases, have caused liver injury; prescription therapy remains the standard when deficiency is confirmed.[32]
Can women use testosterone cream?
Off-label low-dose application under specialist supervision may alleviate hypoactive sexual desire disorder, but female dosing requires markedly lower concentrations and rigorous monitoring for hirsutism or voice changes.[33]
Does site of application matter?
Yes-scrotal or inner-thigh skin yields higher absorption than torso sites; clinicians may select location based on desired pharmacokinetics and patient tolerance.[34]
What precautions reduce skin irritation?
Rotating sites, ensuring complete drying before dressing, and pre-treating with mild topical corticosteroid if prescribed can lessen dermatitis risk.[35]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
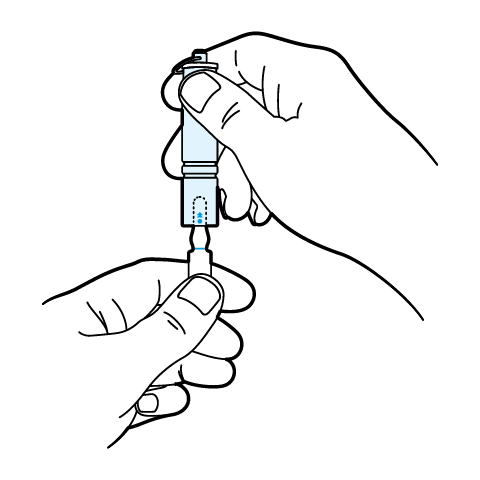
SnapIt Multi-Use Ampoule Opener Instructions
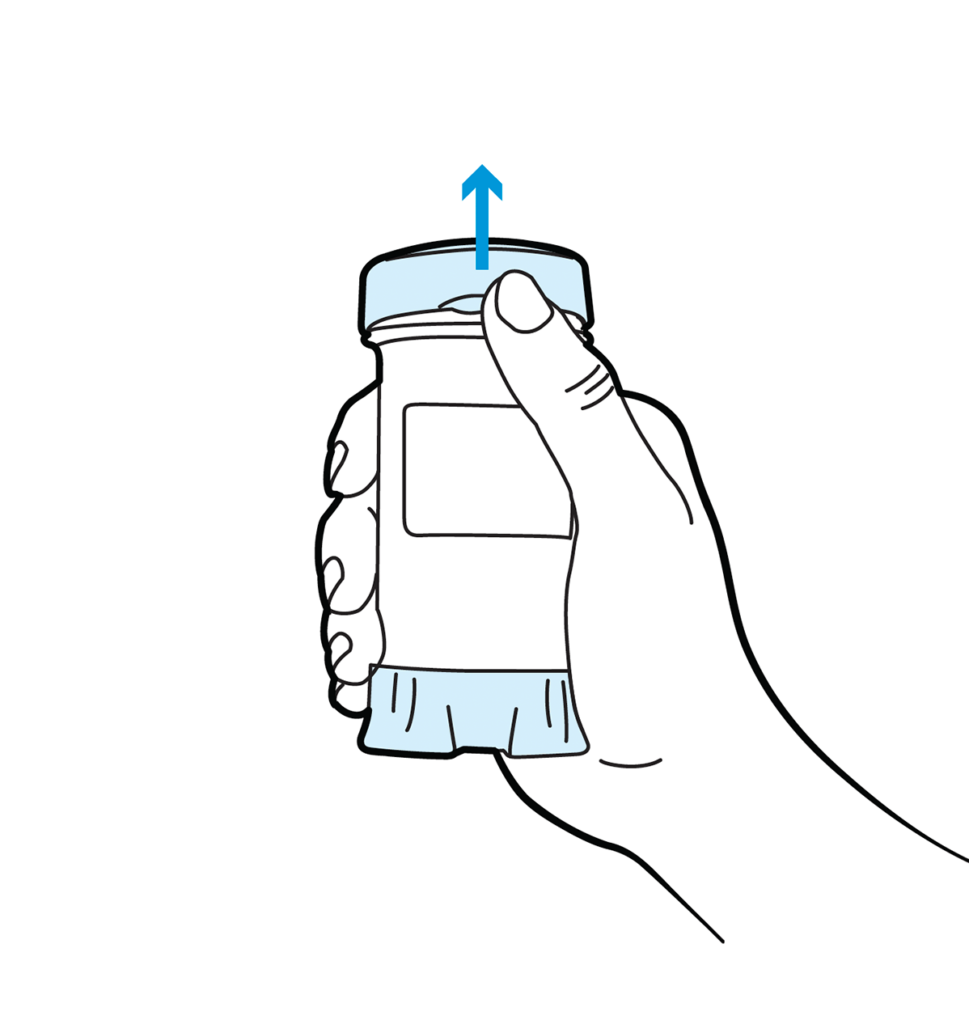
Topi-Click Dispensing Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.






 Testosterone Cypionate Injection
Testosterone Cypionate Injection Testosterone Enanthate Injection
Testosterone Enanthate Injection Nandrolone Decanoate / Testosterone Cypionate / Testosterone Enanthate Injection
Nandrolone Decanoate / Testosterone Cypionate / Testosterone Enanthate Injection Sertraline Tablets
Sertraline Tablets Progesterone Capsules
Progesterone Capsules Progesterone Cream
Progesterone Cream Bi-Est Cream
Bi-Est Cream DHEA Capsules
DHEA Capsules DHEA / Pregnenolone Capsules
DHEA / Pregnenolone Capsules