Product Overview
Testosterone Nasal Gel is a metered-dose, thixotropic hydroalcoholic formulation that delivers bio-identical testosterone across the richly vascular nasal mucosa, allowing systemic absorption without dermal transference or first-pass hepatic metabolism.
In a pivotal phase 3 program involving more than three hundred hypogonadal men, thrice-daily intranasal administration restored average serum testosterone concentrations to the physiologic reference range in over eighty percent of participants while maintaining luteinizing hormone feedback and preserving endogenous spermatogenesis.[1]
Unlike transdermal gels, the nasal route minimizes the risk of unintentionally exposing partners or children because the drug remains confined within the nasal cavity and is rapidly cleared by mucociliary action. Patients in open-label extension phases reported high treatment satisfaction, citing discreet dosing, the absence of application-site odor or residue, and flexibility to travel without refrigeration.[2]
Clinicians have also noted that rapid attainment of steady-state pharmacokinetics facilitates dose titration within the first month of therapy, reducing prolonged periods of sub-therapeutic or supra-physiologic exposure.
A contemporary guideline panel convened by the American Urological Association emphasizes that nasal testosterone may be considered when fertility preservation is a priority because episodic serum peaks appear to exert less chronic suppression on the hypothalamic-pituitary-gonadal axis than long-acting depot injections.
Real-world registry data further suggest that nasal delivery is associated with lower rates of secondary polycythemia and may mitigate dermatologic adverse events common to transdermal vehicles.[2] The product is compounded exclusively under section 503A, meaning each prescription is patient-specific and prepared pursuant to a valid order, thereby allowing customization of dispensing volume or excipient profile in accordance with United States Pharmacopeia <795> standards.
The standard dispensed configuration is a three-milliliter multidosing pen calibrated to deliver ten milligrams (0.05 mL) per actuation. The recommended regimen is one actuation per nostril three times daily-morning, afternoon, and evening-yielding a total of thirty milligrams of testosterone per day.[9]
Patients must prime the device by ten inversions before first use and should blow the nose prior to each dose to maximize mucosal contact.[9] Serum total testosterone should be rechecked at one month; if consistently below three-hundred nanograms per deciliter, clinicians may consider increasing to four times daily or substituting an alternative dosage form.
Therapy should be suspended if levels exceed one-thousand-fifty nanograms per deciliter on two occasions or if hematocrit surpasses fifty-four percent.[10]
Exogenous testosterone traverses nasal epithelial tight junctions primarily by passive diffusion driven by the concentration gradient established immediately after actuation. The nasal cavity offers a surface area approaching one-hundred-and-fifty square centimeters, richly supplied by capillary networks originating from branches of the sphenopalatine and ethmoidal arteries, permitting near-portal-vein bioavailability.[3]
Pharmacokinetic modeling demonstrates a characteristic triple-peak profile: an early maximal concentration approximately one hour post-dose, a mid-interval micro-pulse reflecting endogenous luteinizing hormone stimulation, and a third surge after the next scheduled dose.[4] This pulsatility preserves physiologic diurnal rhythm and partially maintains Leydig cell responsiveness, which may be advantageous for men desiring fertility or avoiding sustained supraphysiologic estradiol conversion.
After entering systemic circulation, testosterone binds predominantly to sex-hormone-binding globulin and albumin; only the free fraction diffuses into target tissues where the androgen receptor mediates genomic and non-genomic actions.
At skeletal muscle and bone, androgen receptor activation promotes protein synthesis, increases alkaline phosphatase activity, and stimulates osteoblastic proliferation. In the central nervous system, aromatization to estradiol modulates mood and libido through hypothalamic dopaminergic pathways. Nasal delivery avoids dermal 5-α-reductase-rich tissue, resulting in a dihydrotestosterone to testosterone ratio closer to endogenous homeostasis, potentially lowering androgenic alopecia and acne incidence.[4]
Compounded testosterone nasal gel must not be prescribed to men with known or suspected carcinoma of the breast or prostate because androgen-responsive neoplastic tissue could proliferate under exogenous stimulation.[5] Additional absolute contraindications include pregnancy, breastfeeding, and hypersensitivity to testosterone or formulation excipients such as benzyl alcohol.
Therapy is inadvisable in individuals with uncontrolled severe sleep apnea, untreated polycythemia (hematocrit > 54 %), or poorly controlled heart failure, as androgen-induced erythrocytosis and fluid retention may exacerbate cardiopulmonary strain.[5] Clinicians should also exercise caution in patients with thrombophilia or a history of venous thromboembolism, given post-marketing analyses suggesting a temporal association between testosterone therapy and thrombotic events in susceptible populations.
Baseline evaluation should encompass digital rectal examination, prostate-specific antigen measurement, hematocrit, lipid profile, and assessment of cardiovascular risk factors, repeating surveillance at three to six months and annually thereafter.[5]
Testosterone is a substrate of hepatic CYP3A4 and an inhibitor of intestinal P-glycoprotein; therefore, co-administration with strong CYP3A4 inhibitors such as ketoconazole or ritonavir may elevate systemic androgen exposure, increasing the likelihood of edema and gynecomastia.[6] Conversely, agents that induce CYP3A4 (e.g., rifampin, carbamazepine) could lower therapeutic levels, risking persistent hypogonadal symptoms.
Anticoagulants including warfarin and direct-acting oral anticoagulants warrant vigilant international normalized ratio or anti-Xa monitoring, as androgen-mediated reductions in clotting factors may potentiate bleeding.[6] Concomitant corticosteroid therapy can amplify sodium and water retention, necessitating periodic blood pressure and electrolyte assessment.
Sympathomimetic decongestants such as oxymetazoline administered intranasally thirty minutes before testosterone reduce bioavailability by approximately three percent, a change that is not clinically meaningful but may warrant consistency in timing to preserve pharmacokinetic predictability.[6]
In controlled trials, the most common local reactions were mild nasopharyngitis, rhinorrhea, nasal discomfort, and transient epistaxis.[7] Systemic adverse events mirrored those of other testosterone formulations and included increased prostate-specific antigen, acne, polycythemia, and modest rises in systolic blood pressure.
Importantly, the incidence of erythrocytosis was under two percent-significantly lower than rates reported with injectable esters-likely reflecting lower peak-trough variability.[7] Long-term extensions up to twelve months have not demonstrated clinically significant deterioration in olfactory testing or nasal endoscopy scores.
Nonetheless, patients should be counseled to report persistent congestion, crusting, or changes in smell. Post-marketing surveillance continues to investigate rare but serious events such as myocardial infarction and stroke; current evidence remains inconclusive, and prescribers are advised to individualize therapy based on comprehensive cardiovascular risk assessment.[7]
Testosterone is teratogenic and classified as pregnancy category X; exposure during gestation can masculinize the external genitalia of a female fetus and disrupt endocrine development.[8] Although nasal administration reduces partner transfer risk relative to topical gels, trace amounts detected in seminal fluid and sweat necessitate barrier contraception when pregnancy is possible.
Testosterone therapy is not considered effective contraception and ovulation may persist in transgender or gender-diverse persons using androgen therapy.[8] If pregnancy occurs, the medication should be discontinued immediately, and obstetric consultation sought to evaluate potential fetal virilization.
When compounded under 503A, testosterone nasal gel is a non-sterile, aqueous preparation with a recommended beyond-use date of ninety days when stored at controlled room temperature not exceeding twenty-five degrees Celsius.[11]
The pen should remain tightly capped to prevent ethanol evaporation and viscosity changes that could affect metered dosing accuracy. Patients should avoid freezer storage, extreme heat, or prolonged sunlight exposure, as such conditions may precipitate crystallization or degradation of the active ingredient.
- Gronski, M. A., Grober, E. D., Gottesman, I. S., Ormsby, R. W., & Bryson, N. (2019). Efficacy of nasal testosterone gel stratified by baseline endogenous testosterone levels. Journal of the Endocrine Society, 3(9), 1652-1662. https://doi.org/10.1210/js.2019-00183
- Mulhall, J. P., Trost, L. W., Brannigan, R. E., et al. (2018). Evaluation and management of testosterone deficiency: AUA guideline. Journal of Urology, 200(2), 423-432. https://doi.org/10.1016/j.juro.2018.03.115
- Touitou, E., & Illum, L. (2025). The review of nasal drug delivery system: Strategies to enhance intranasal absorption. International Journal of Pharmaceutics, 676, 120162. https://doi.org/10.1016/j.ijpharm.2025.120162
- Shrewsbury, S. B., Davies, G., & McConnachie, L. (2022). The pharmacokinetics of drug delivery to the upper nasal space. Medical Research Archives, 10(9). https://doi.org/10.18103/mra.v10i9.2971
- U.S. Food and Drug Administration. (2024). Natesto (testosterone) nasal gel prescribing information. Drug Labeling Archive. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022219s014lbl.pdf
- Altıntaş Aykan, D., & Aykan, A. Ç. (2021). Interaction of cardiovascular drugs with testosterone therapy. Journal of Men’s Health, 17(2), 25-31. https://doi.org/10.31083/jomh.2021.005
- NATESTO Healthcare Professional Site. (2025). Safety profile of testosterone nasal gel. Acerus Pharmaceuticals. https://www.natestohcp.com/safety/
- Centers for Disease Control and Prevention. (2024). Testosterone use and risk for pregnancy. U.S. Selected Practice Recommendations. https://www.cdc.gov/contraception/hcp/usspr/testosterone-pregnancy-risk.html
- Drugs.com. (2025). Natesto nasal gel dosage guide. Medical Review March 31 2025. https://www.drugs.com/dosage/natesto-nasal-gel.html
- NATESTO Healthcare Professional Site. (2025). Dosing and prescribing information. Acerus Pharmaceuticals. https://www.natestohcp.com/dosing-and-prescribing/
- United States Pharmacopeia. (2022). Compounding standards and beyond-use dates fact sheet. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf
- Clarke, G., & Brooks, R. (2019). Testosterone replacement therapy: Patient-reported outcomes. Verywell Health. https://www.verywellhealth.com/testosterone-replacement-therapy-4581254
- Lancet Healthy Longevity Editorial Board. (2022). Adverse cardiovascular events in men during testosterone therapy. The Lancet Healthy Longevity, 3(11), e742-e744. https://doi.org/10.1016/S2666-7568(22)00096-4
- Udroiu, A., Roland, M., & Franz, M. (2022). Testosterone supplementation and cardiovascular risk: A review. Romanian Journal of Internal Medicine, 60(2), 88-96. https://doi.org/10.2478/rjim-2022-0022
- Nackeeran, S., et al. (2022). Effect of testosterone route on hematocrit changes: Systematic review. Journal of Urology, 207(1), 44-51. https://doi.org/10.1097/JU.0000000000002193
- Sciencedirect Newsroom. (2024). Association between testosterone therapy and cardiovascular outcomes. Pharmacology & Therapeutics, 257, 107999. https://doi.org/10.1016/j.pharmthera.2024.107999
- American Society of Health-System Pharmacists. (2024). Crosswalk of guidance and standards for assigning beyond-use dates in compounding. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/compounding/docs/Crosswalk-of-Guidance-and-Standards-for-Managing-BUDs.pdf
- Natesto Full Prescribing Information. (2020). Section 11 Description and composition. Acerus Pharmaceuticals. https://www.natesto.com/pdfs/natesto-full-prescribing-information.pdf
- University of Utah Health. (2018). Teratogenicity and drug-related pregnancy risks. https://physicians.utah.edu/sites/g/files/zrelqx276/files/media/documents/2021/2018.5.18-teratogenicity-drug-related-pregnancy-risks.pdf
- European Review for Medical and Pharmacological Sciences. (2023). Mechanisms and solutions for nasal drug delivery. https://www.europeanreview.org/wp/wp-content/uploads/72-81.pdf
- Howard, J. (2023, June 13). Testosterone treatments and heart risk when patients are monitored. Time Magazine. https://time.com/6287805/testosterone-treatments-heart-risk/
How quickly will energy and libido improve after starting nasal testosterone?
Most men report perceptible increases in vitality and sexual desire within two to four weeks, coinciding with normalization of serum levels.[12]
Does intranasal dosing carry the same cardiovascular risk as injections?
Observational meta-analyses indicate no significant elevation in major adverse cardiac events when testosterone levels are maintained within physiologic limits and patients are appropriately monitored.[13]
Can the pen be shared between patients?
No; device sharing risks cross-contamination and violates compounding regulations mandating patient-specific dispensing.[14]
Will the medication worsen benign prostatic hyperplasia?
Androgens may exacerbate lower urinary tract symptoms in susceptible individuals; routine prostate assessment helps balance benefits and risks.[15]
Is nasal testosterone suitable for bodybuilding or performance enhancement?
Off-label, non-therapeutic use is illegal and increases the likelihood of adverse cardiovascular and reproductive outcomes.[16]
What happens if a dose is missed?
Administer the dose as soon as remembered unless it is nearly time for the next scheduled administration; doubling up to compensate is discouraged.[17]
Are there preservatives that could trigger allergies?
The formulation contains benzyl alcohol and polyethylene glycol; hypersensitivity is rare but warrants discontinuation if severe rhinitis develops.[18]
How is treatment monitored long-term?
Providers typically reassess testosterone, hematocrit, PSA, liver enzymes, and blood pressure at three months, six months, and annually thereafter.[19]
Can women ever receive testosterone nasal gel?
Current evidence and labeling restrict therapy to men with documented hypogonadism; research in female sexual dysfunction is exploratory and unapproved.[20]
Does insurance cover compounded nasal testosterone?
Reimbursement varies; patients should verify benefits and may provide documentation of treatment necessity when required.[21]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
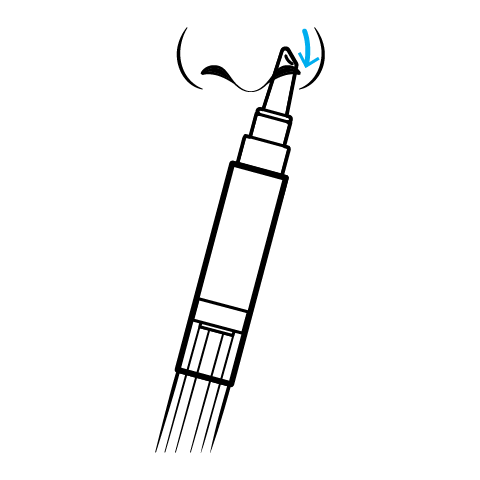
Testosterone Nasal Gel Application Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Testosterone Cypionate Injection
Testosterone Cypionate Injection Testosterone Enanthate Injection
Testosterone Enanthate Injection Nandrolone Decanoate / Testosterone Cypionate / Testosterone Enanthate Injection
Nandrolone Decanoate / Testosterone Cypionate / Testosterone Enanthate Injection Sertraline Tablets
Sertraline Tablets Sildenafil / Tadalafil ODT
Sildenafil / Tadalafil ODT Sildenafil / Oxytocin ODT
Sildenafil / Oxytocin ODT Tadalafil ODT
Tadalafil ODT Sildenafil ODT
Sildenafil ODT Enclomiphene Citrate Capsules
Enclomiphene Citrate Capsules