Product Overview
Tirzepatide / Niacinamide Injection is a compounded formulation that combines the dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide with the B-vitamin derivative niacinamide. This compounded injection is available exclusively through our 503A compounding pharmacy pursuant to a patient-specific prescription.
Physicians may prescribe this compounded medication for patients who, the physician determines, may benefit from the combined effects of both active ingredients. The compounded medication is prepared in a sterile environment according to compounding standards. The injection is available in multiple dosage strengths to accommodate physician determinations of patient need, with formulations that may include 17 mg/mL tirzepatide with 2 mg/mL niacinamide in 4 mL vials, 8 mg/mL tirzepatide with 2 mg/mL niacinamide in 2.5 mL vials, and 17 mg/mL tirzepatide with 2 mg/mL niacinamide in 2 mL vials.
Patients receiving this medication should understand that it is a compounded preparation, meaning it is specifically prepared for individual use and is not a commercially manufactured pharmaceutical product. Compounded medications are not FDA-approved medications, and FDA does not review compounded medications for safety or efficacy.
Healthcare providers typically prescribe Tirzepatide / Niacinamide Injection for patients who the physician has determined may benefit from the metabolic effects of tirzepatide while potentially gaining additional benefits from niacinamide supplementation. The formulation process ensures that both active ingredients maintain their stability and when combined in the injection vehicle.
Patients and healthcare providers should be aware that this is a compounded medication tailored for individual patients and should only be used under appropriate medical supervision. The compounded medication is prepared in accordance compounding guidelines. Regular monitoring and follow-up with prescribing healthcare providers is essential to optimize therapeutic outcomes and ensure appropriate response to treatment.
The dosage and administration of Tirzepatide / Niacinamide Injection requires careful individualization based on patient characteristics, treatment goals, and tolerance to therapy. The medication is available in multiple formulations to accommodate different patient needs and treatment protocols, with dosing typically following a gradual titration approach to optimize therapeutic benefits while minimizing adverse effects.
The available formulations include 17 mg/mL tirzepatide with 2 mg/mL niacinamide in 4 mL vials, 8 mg/mL tirzepatide with 2 mg/mL niacinamide in 2.5 mL vials, and 17 mg/mL tirzepatide with 2 mg/mL niacinamide in 2 mL vials. Physicians determine the most appropriate formulation based on the specific patients’ needs.
Administration should occur via subcutaneous injection, with recommended injection sites including the abdomen, thigh, or upper arm. Patients should be instructed to rotate injection sites to minimize the risk of injection site reactions and to avoid injecting into areas that are tender, bruised, red, or hard. The injection should be administered once weekly on the same day each week, though the specific time of day can be flexible based on patient preference and lifestyle factors.
Proper injection technique is crucial for optimal absorption and to minimize injection site reactions. Patients should use aseptic technique, including proper hand hygiene and skin preparation. The injection should be administered slowly and steadily, and the needle should remain in place for several seconds after injection to ensure complete delivery of the dose.
Dose adjustments may be necessary based on patient response, tolerance, and individual treatment goals, as determined by the patient’s physician. Patients experiencing significant gastrointestinal side effects may benefit from temporary dose reduction or slowing the titration schedule. Conversely, patients who are tolerating the medication well but not achieving desired therapeutic outcomes may benefit from dose increases within the recommended range.
Patients should receive comprehensive education about proper injection technique, dose timing, storage requirements, and what to do if doses are missed. They should also be instructed about signs and symptoms that warrant dose adjustment or medical evaluation, including severe gastrointestinal side effects, signs of hypoglycemia, or unusual symptoms that may indicate adverse reactions.
Healthcare providers should work closely with patients to optimize dosing based on individual response patterns, treatment goals, and tolerance. Regular follow-up appointments should include assessment of therapeutic response, monitoring for adverse effects, and evaluation of the need for dose adjustments. This collaborative approach helps ensure that patients receive the maximum benefit from therapy while minimizing the risk of adverse effects.
Tirzepatide / Niacinamide Injection has several important contraindications that must be carefully considered before initiating therapy. These contraindications stem from the pharmacological properties of both active ingredients and the potential risks associated with their use in certain patient populations or medical conditions.
The most significant contraindication for this combination injection is a known hypersensitivity or allergy to tirzepatide, niacinamide, or any component of the formulation. Patients who have previously experienced allergic reactions to GLP-1 receptor agonists or GIP receptor agonists should be evaluated carefully, as cross-reactivity may occur. Allergic reactions to incretin-based therapies can range from mild local reactions to severe systemic hypersensitivity, including anaphylaxis in rare cases. Similarly, while niacinamide is generally well-tolerated, individuals with known sensitivities to B-vitamins or nicotinic acid derivatives should exercise caution.
Patients with a personal or family history of medullary thyroid carcinoma represent another critical contraindication for tirzepatide-containing formulations. Incretin receptor agonists, including tirzepatide, may potentially increase the risk of thyroid C-cell tumors based on findings in animal studies. The potential risk necessitates avoiding use in patients with this specific cancer history. Additionally, patients with Multiple Endocrine Neoplasia syndrome type 2 should not receive tirzepatide due to the theoretical increased risk of medullary thyroid carcinoma associated with this genetic condition.
Severe gastroparesis or significant gastric motility disorders constitute another important contraindication. Since tirzepatide may slow gastric emptying as part of its mechanism of action, patients with pre-existing severe gastroparesis could experience worsening of symptoms, including nausea, vomiting, and potential complications related to delayed gastric emptying. This effect could be particularly problematic in patients with diabetic gastroparesis or other conditions affecting gastric motility.
Patients with a history of severe gastrointestinal disease, particularly those with inflammatory bowel disease, severe gastroesophageal reflux disease, or history of pancreatitis, require careful evaluation before considering this therapy. While not absolute contraindications in all cases, these conditions may increase the risk of gastrointestinal adverse effects or complications. Acute pancreatitis has been reported with incretin-based therapies, and patients with a history of pancreatitis may be at increased risk for recurrence.
Pregnancy represents a significant contraindication for this combination therapy.
Patients with severe kidney disease or end-stage renal disease may not be appropriate candidates for this therapy, particularly if they have significant fluid retention or are prone to dehydration. While tirzepatide is not primarily eliminated through the kidneys, the gastrointestinal effects of the medication, including potential nausea, vomiting, and diarrhea, could lead to dehydration and electrolyte imbalances that may be particularly problematic in patients with compromised kidney function.
Severe liver disease or hepatic impairment may also represent a contraindication, particularly if associated with significant metabolic dysfunction or impaired drug metabolism. While niacinamide is generally safe in patients with liver disease, high doses or compromised hepatic function could potentially affect the metabolism and clearance of both active components.
Patients with active eating disorders, particularly those involving restrictive eating patterns or purging behaviors, may not be appropriate candidates for this therapy due to the appetite-suppressing effects of tirzepatide and the potential for exacerbating unhealthy eating behaviors. The significant weight loss effects of tirzepatide could be problematic in patients with anorexia nervosa or other conditions where further weight loss would be harmful.
Additionally, patients taking medications with significant drug interactions or those with conditions that could be exacerbated by changes in glucose levels or gastric emptying should be carefully evaluated. The decision to use this combination therapy should always involve careful consideration of the patient’s complete medical history, current medications, and individual risk factors.
Tirzepatide / Niacinamide Injection may interact with various medications, supplements, and medical procedures, requiring careful consideration and potential adjustments to concurrent therapies. Understanding these interactions is crucial for safe and effective use of this combination formulation.
The most clinically significant interactions involve medications that affect blood glucose levels. Tirzepatide’s glucose-lowering effects may be additive with other antidiabetic medications, potentially increasing the risk of hypoglycemia when used concurrently with insulin, sulfonylureas, meglitinides, or other glucose-lowering agents. Patients taking insulin may require dose reductions to prevent hypoglycemic episodes, particularly as tirzepatide therapy is initiated and titrated. The timing and magnitude of insulin dose adjustments should be carefully monitored and individualized based on blood glucose patterns and patient response.
Sulfonylureas and meglitinides present particular interaction concerns due to their mechanism of stimulating insulin release independent of glucose levels. When combined with tirzepatide, which provides glucose-dependent insulin stimulation, the risk of hypoglycemia may be significantly increased. Healthcare providers may need to reduce doses of these medications or consider alternative antidiabetic agents with lower hypoglycemia risk when initiating tirzepatide therapy.
The gastric emptying effects of tirzepatide may significantly impact the absorption and effectiveness of oral medications that require specific timing or absorption patterns. Medications with narrow therapeutic windows, such as warfarin, digoxin, or certain antiepileptic drugs, may require more frequent monitoring when tirzepatide is initiated, as delayed gastric emptying could affect absorption rates and peak concentration timing. This is particularly important for medications where consistent absorption is critical for therapeutic effectiveness.
Oral contraceptives may be affected by the gastric motility changes associated with tirzepatide therapy. While the clinical significance remains unclear, women relying on oral contraceptives should be advised about potential interactions and may need to consider additional contraceptive methods or more frequent monitoring to ensure continued effectiveness. The timing of oral contraceptive administration in relation to tirzepatide injections may also require adjustment.
Niacinamide may interact with certain medications that affect niacin metabolism or that have overlapping effects on lipid metabolism. While niacinamide generally has fewer interactions than nicotinic acid, concurrent use with other B-vitamin supplements or medications affecting vitamin metabolism should be monitored. Patients taking large doses of other B-vitamins or multivitamin supplements may need evaluation for potential additive effects or imbalances.
Medications that affect gastric pH or gastric emptying may have enhanced or reduced effects when used concurrently with tirzepatide. Proton pump inhibitors, H2 receptor antagonists, and prokinetic agents may have altered effectiveness due to tirzepatide’s effects on gastric function. Patients using these medications may require monitoring for changes in symptom control or therapeutic effectiveness.
Alcohol consumption represents an important interaction consideration, as alcohol may enhance the glucose-lowering effects of tirzepatide and increase the risk of hypoglycemia. Additionally, alcohol may exacerbate gastrointestinal side effects such as nausea and vomiting. Patients should be counseled about moderate alcohol consumption and the importance of monitoring blood glucose levels when consuming alcohol while on tirzepatide therapy.
Certain antibiotics, particularly those that may affect gastrointestinal function or glucose metabolism, require careful monitoring when used concurrently with this combination therapy. Fluoroquinolones, which may affect glucose homeostasis, and antibiotics that significantly alter gastrointestinal flora may interact with the metabolic effects of both tirzepatide and niacinamide.
Procedures involving contrast agents or requiring specific medication timing may need special consideration in patients receiving Tirzepatide / Niacinamide Injection. The delayed gastric emptying effects could impact pre-procedure fasting requirements or the timing of pre-procedure medications. Medical procedures requiring specific glycemic control may also need adjusted monitoring protocols.
Supplements containing chromium, alpha-lipoic acid, or other compounds marketed for glucose control may have additive effects with tirzepatide, potentially increasing hypoglycemia risk. Patients should inform healthcare providers about all supplements and over-the-counter products they are using to assess for potential interactions.
The interaction potential may also extend to herbal supplements that affect glucose metabolism or gastrointestinal function. Products containing bitter melon, cinnamon, fenugreek, or other herbs with purported glucose-lowering effects may enhance the hypoglycemic risk when combined with tirzepatide. Similarly, herbs that affect gastric motility or gastrointestinal function may interact with tirzepatide’s gastric effects.
Patients should be advised to maintain consistent timing of all medications when possible and to inform all healthcare providers about their use of Tirzepatide / Niacinamide Injection when receiving treatment for other conditions or when new medications are prescribed. Regular monitoring and communication with healthcare providers can help identify and manage potential interactions before they become clinically significant.
Tirzepatide / Niacinamide Injection may cause various side effects ranging from common, generally mild reactions to rare but potentially serious adverse events. Understanding the side effect profile helps patients and healthcare providers make informed decisions about treatment and enables early recognition and management of adverse reactions.
The most commonly observed side effects are gastrointestinal in nature, primarily related to tirzepatide’s effects on gastric emptying and incretin receptor activation. Nausea represents the most frequently reported adverse reaction, affecting a significant proportion of patients, particularly during the initial weeks of therapy or following dose increases. This nausea is typically mild to moderate in severity and often improves as patients become accustomed to the medication. The nausea may be accompanied by decreased appetite, which, while potentially beneficial for weight management goals, can occasionally be excessive and require monitoring.
Vomiting occurs less frequently than nausea but can be problematic when it does occur, particularly if it leads to dehydration or interferes with nutrition and medication absorption. Patients experiencing persistent vomiting should be evaluated for dose adjustment or temporary discontinuation. Diarrhea is another commonly reported gastrointestinal side effect that may range from mild loose stools to more significant episodes that could lead to fluid and electrolyte imbalances if severe or prolonged.
Abdominal pain and discomfort may occur, often related to the gastric emptying effects of tirzepatide. This pain is typically described as mild to moderate and may be associated with feelings of fullness or bloating. Constipation, while less common than diarrhea, may also occur and can be particularly problematic in patients with pre-existing constipation issues.
Injection site reactions represent another category of commonly observed side effects. These may include redness, swelling, itching, or mild pain at the injection site. Most injection site reactions are mild and resolve spontaneously within a few days. Proper injection technique and site rotation can help minimize these reactions. Occasionally, patients may develop small nodules or areas of firmness at injection sites, which typically resolve over time.
Fatigue and weakness may occur, particularly during the initial treatment period as the body adjusts to the metabolic effects of the medication. Some patients report feeling tired or having reduced energy levels, which may be related to changes in eating patterns, blood glucose levels, or the direct effects of the medication on metabolism.
Headache is reported by some patients and may be related to changes in blood glucose levels, dehydration from gastrointestinal side effects, or direct pharmacological effects. Most headaches are mild and respond to standard headache management approaches, but persistent or severe headaches should be evaluated.
Dizziness may occur, particularly in patients who experience significant changes in blood glucose levels or who become dehydrated due to gastrointestinal side effects. This dizziness is usually mild and transient but can be concerning if it affects daily activities or increases fall risk.
More serious but less common side effects require immediate medical attention. Severe abdominal pain, particularly if accompanied by nausea, vomiting, and fever, may indicate pancreatitis, a rare but serious complication that has been reported with incretin-based therapies. Patients should be instructed to seek immediate medical care if they experience severe, persistent abdominal pain that may radiate to the back.
Allergic reactions, while uncommon, can range from mild skin reactions to severe systemic hypersensitivity. Mild allergic reactions may present as skin rash, itching, or hives. More severe reactions could include difficulty breathing, swelling of the face, lips, tongue, or throat, which would require immediate emergency medical treatment.
Kidney problems, while rare, may occur, particularly in patients who become severely dehydrated due to gastrointestinal side effects. Signs of kidney problems may include changes in urination patterns, swelling in the legs or feet, or unusual fatigue. Patients should maintain adequate hydration and seek medical attention if they notice concerning changes.
Gallbladder problems, including gallstones or gallbladder inflammation, have been reported with significant weight loss and may occur in patients using tirzepatide. Symptoms may include severe abdominal pain, particularly in the upper right area, nausea, vomiting, and fever.
Hypoglycemia, while less common with tirzepatide alone due to its glucose-dependent mechanism, may occur, particularly in patients taking concurrent glucose-lowering medications. Symptoms of hypoglycemia include shakiness, sweating, confusion, rapid heartbeat, and in severe cases, loss of consciousness.
Changes in vision, while uncommon, may occur due to fluctuations in blood glucose levels or fluid shifts. Most vision changes are temporary and resolve as glucose levels stabilize, but persistent vision changes should be evaluated by an eye care professional.
Niacinamide-related side effects are generally mild and uncommon at the doses used in this formulation. Occasionally, patients may experience mild gastrointestinal upset, skin flushing, or headache related to the niacinamide component, though these are typically less pronounced than with higher doses of nicotinic acid.
Some patients may experience mood changes or anxiety, which could be related to changes in eating patterns, blood glucose levels, or direct pharmacological effects. These mood changes are typically mild and may improve as patients adjust to the medication.
It is important for patients to report any unusual or concerning symptoms to their healthcare provider, as individual responses to medication can vary significantly. Early recognition and appropriate management of side effects can often allow continued treatment with dose adjustments or supportive measures rather than requiring discontinuation of therapy.
If pregnancy occurs while using this medication, you should immediately contact your healthcare provider to discuss whether you should continue using this compounded medication.
You should follow your healthcare providers’ instructions regarding when this medication can be resumed, if discontinued, and whether this medication may be used during breastfeeding.
Proper storage of Tirzepatide / Niacinamide Injection is critical for maintaining the stability and potency of this compounded medication. The storage requirements for this formulation reflect the need to preserve the integrity of both active ingredients while preventing contamination and degradation that could compromise therapeutic effectiveness or patient safety.
The medication should be stored under refrigeration at temperatures between 36°F and 46°F (2°C to 8°C) until ready for use. Refrigeration is essential for maintaining the stability of tirzepatide, which is a peptide medication that can degrade at higher temperatures. The cold chain should be maintained from the time of compounding through dispensing and patient storage to ensure optimal medication quality. Patients should store the medication in the main body of the refrigerator rather than in door compartments, where temperature fluctuations may be more significant.
The medication must be protected from light exposure, as both active ingredients may be sensitive to light-induced degradation. The vials should be kept in their original packaging or stored in a light-protective container when not in use. Direct sunlight and bright artificial lighting should be avoided, and the medication should not be left exposed to room lighting for extended periods.
Freezing must be strictly avoided, as freezing can cause irreversible damage to the peptide structure of tirzepatide and may alter the formulation characteristics. If the medication accidentally freezes, it should not be used and should be disposed of properly. Patients should be educated about the importance of preventing freezing and should take precautions when transporting the medication, particularly during cold weather.
Before administration, the medication may be removed from refrigeration and allowed to reach room temperature, which can help reduce injection site discomfort. However, the medication should not be left at room temperature for extended periods. If removed from refrigeration, it should be used within a reasonable timeframe, typically within 2-4 hours, and should not be returned to refrigeration after reaching room temperature for injection.
Each vial should be inspected visually before each use for any signs of particulate matter, discoloration, cloudiness, or other changes in appearance. The solution should be clear and colorless to slightly yellow. Any visible particles, cloudiness, color changes, or other abnormalities indicate potential contamination or degradation, and the vial should not be used. Patients should be educated about what normal medication appearance looks like and instructed to contact their pharmacy or healthcare provider if they notice any changes.
The medication should be kept in its original vial with the original labeling intact. Transfer to other containers is not recommended as it may compromise sterility and could lead to dosing errors. The rubber stopper should not be removed, and the vial should only be accessed using appropriate sterile technique with sterile needles and syringes.
Storage should be in a location that is secure and out of reach of children and pets. The medication should be stored in an area where temperature and humidity are relatively stable, away from heat sources, and in a location where accidental damage or contamination is unlikely. Medicine cabinets in bathrooms may not be ideal due to humidity and temperature fluctuations associated with shower use.
During transport, appropriate measures should be taken to maintain the cold chain. This may include using insulated containers with ice packs or cool packs, though direct contact between the medication and ice should be avoided to prevent freezing. For longer trips or shipping, specialized pharmaceutical transport containers may be necessary to ensure temperature stability.
Multi-dose vials, once accessed with a needle, have additional storage considerations related to sterility maintenance. The rubber stopper should be cleaned with alcohol before each access, and sterile technique should be maintained throughout the withdrawal process. The vial should be dated when first accessed, and the 28-day rule for multi-dose vials typically applies unless otherwise specified by the compounding pharmacy.
If traveling, patients should plan ahead to ensure appropriate storage conditions can be maintained. This may include arranging for refrigeration at destinations, using portable cooling systems, or coordinating with healthcare providers or pharmacies at travel destinations for medication storage if necessary.
Disposal of unused or expired medication should follow appropriate pharmaceutical waste disposal guidelines. The medication should not be disposed of in household trash or flushed down toilets or drains, as this can contribute to environmental contamination. Many pharmacies and healthcare facilities offer medication disposal services, or community disposal events may be available. Patients should contact their pharmacy or local waste management authority for guidance on proper disposal methods in their area.
Any concerns about storage conditions, medication appearance, or questions about proper storage should be directed to the compounding pharmacy or healthcare provider. Maintaining proper storage conditions is essential for ensuring the medication remains safe and effective throughout its intended use period.
What is the difference between this compounded injection and commercially available tirzepatide products?
Tirzepatide / Niacinamide Injection is a compounded medication that combines tirzepatide with niacinamide, whereas commercially available products contain only tirzepatide. Compounded medications are prepared individually for specific patients based on physician prescriptions of patient need. Compounded medications may offer dosing flexibility or combinations not available in commercial products. Compounded medications are not FDA-approved drugs. FDA does not review compounded medications for safety or efficacy.
Can I take this medication with my current diabetes medications?
This medication may be used with other diabetes medications, but dosing adjustments are often necessary to prevent hypoglycemia. The combination with insulin, sulfonylureas, or other glucose-lowering medications requires careful monitoring and potential dose reductions of existing medications. Your healthcare provider will need to review all current medications and may need to adjust doses or timing as tirzepatide therapy is initiated and titrated. Regular blood glucose monitoring becomes even more important when combining multiple glucose-lowering therapies, and you should work closely with your healthcare team to optimize your medication regimen.
What should I do if I experience severe nausea or vomiting?
Mild to moderate nausea is common when starting this medication and often improves with time. However, severe or persistent nausea and vomiting require medical attention. If you experience severe symptoms, contact your healthcare provider, as dose reduction or temporary discontinuation may be necessary. In the meantime, try eating smaller, more frequent meals, avoiding fatty or spicy foods, and staying well-hydrated. If vomiting prevents you from keeping fluids down or if you develop signs of dehydration, seek immediate medical care. Your healthcare provider may recommend anti-nausea medications or adjust your dosing schedule to improve tolerance.
Is it safe to drink alcohol while using this medication?
Alcohol consumption should be approached with caution while using this medication. Alcohol may increase the risk of hypoglycemia and can exacerbate gastrointestinal side effects such as nausea and vomiting. If you choose to consume alcohol, do so in moderation and with food to help minimize these risks. Monitor your blood glucose levels more frequently when consuming alcohol, and be aware of symptoms of low blood sugar. Discuss your alcohol consumption habits with your healthcare provider to determine what level of consumption, if any, is appropriate for your individual situation.
Can I continue this medication if I’m planning to become pregnant?
This medication is not recommended during pregnancy due to insufficient safety data and potential risks to fetal development. If you are planning to become pregnant, you should discuss alternative treatment options with your healthcare provider well before conception. Effective contraception is recommended while using this medication to prevent unintended pregnancy. If you become pregnant while taking this medication, contact your healthcare provider immediately to discuss whether this medication can be continued.
What happens if I miss a dose?
If you miss a dose, administer it as soon as possible within 4 days of the scheduled time. If more than 4 days have passed, skip the missed dose and resume your regular weekly schedule with the next planned injection. Do not double dose or administer two injections within 3 days of each other to make up for a missed dose. Missing occasional doses is not typically harmful, but try to maintain consistency for optimal therapeutic benefits. If you frequently forget doses, discuss strategies with your healthcare provider to improve adherence, such as setting reminders or adjusting your injection day.
Are there any dietary restrictions I should follow while taking this medication?
While there are no absolute dietary restrictions, certain dietary approaches may help optimize the medication’s benefits and minimize side effects. Eating smaller, more frequent meals may help reduce gastrointestinal side effects. Avoiding high-fat, greasy, or very spicy foods may also help minimize nausea and digestive discomfort. Focus on balanced nutrition with adequate protein, as the appetite suppression effects may sometimes lead to insufficient protein intake. Stay well-hydrated, especially if experiencing any gastrointestinal side effects. Your healthcare provider or a registered dietitian can provide personalized nutrition guidance to complement your treatment.
How should I rotate injection sites, and what sites are recommended?
Recommended injection sites include the abdomen (avoiding the area around the navel), the front and sides of the thighs, and the back of the upper arms. Rotate between different sites and different areas within each site to prevent injection site reactions and ensure consistent absorption. Keep a record of injection sites to help with systematic rotation. Avoid injecting into areas that are tender, bruised, red, hard, or scarred. Clean the injection site with alcohol before each injection and allow the area to dry completely. Use each injection site only once per week, giving the area time to recover between injections.
What should I tell other healthcare providers about this medication?
Inform all healthcare providers that you are taking Tirzepatide / Niacinamide Injection, including dentists, specialists, emergency room physicians, and pharmacists. This is particularly important before any medical procedures, surgeries, or when starting new medications. The medication can affect blood glucose levels and gastric emptying, which may be relevant for various medical procedures. Carry a list of all your medications, including this compounded injection, and ensure it’s included in your medical records. If you require emergency care, make sure emergency personnel are aware of your medication use, as it may affect treatment decisions and blood glucose management.
Administration Instructions
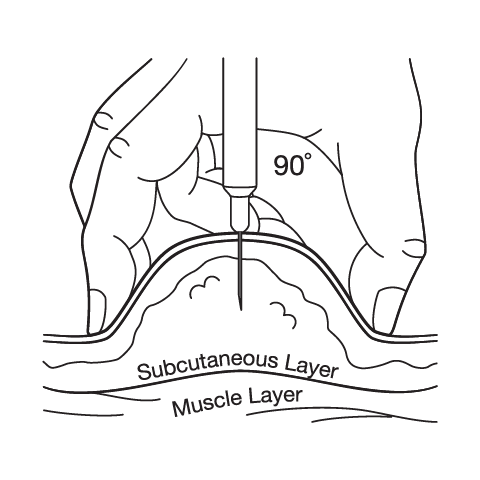
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.




 Semaglutide / Cyanocobalamin Injection
Semaglutide / Cyanocobalamin Injection Tirzepatide ODT
Tirzepatide ODT Sermorelin Acetate Injection
Sermorelin Acetate Injection Bella Capsules
Bella Capsules Lipo-C Injection
Lipo-C Injection Lipo-B Injection
Lipo-B Injection Phentermine HCl Capsules
Phentermine HCl Capsules Lipo Injection
Lipo Injection Sermorelin Acetate ODT
Sermorelin Acetate ODT Ondansetron ODT
Ondansetron ODT