Product Overview
Semaglutide
Semaglutide / Cyanocobalamin Injection is a compounded medication that combines two distinct therapeutic agents to address metabolic health and nutritional support in a single formulation. This compounded preparation is available exclusively through our 503A compounding pharmacy pursuant to a patient-specific prescription and is tailored for individual patients based on their specific needs as determined by the prescribing physician.
As a compounded medication tailored for individual patients, Semaglutide / Cyanocobalamin Injection is prepared in sterile injection form. The medication is formulated in various strengths to accommodate physician determinations of patient need, with concentrations ranging from 1 mg/mL to 5 mg/mL of semaglutide combined with 0.5 mg/mL of cyanocobalamin, available in both 1 mL and 2.5 mL vial sizes. This flexibility in dosing options allows healthcare providers to customize treatment regimens based on individual patient responses, tolerability, and therapeutic goals. By combining these agents in a single injection, the formulation may improve patient adherence to treatment protocols while potentially reducing the burden side effects or and multiple injections or medications, as determined by necessary by the prescribing physician.
It is essential to understand that Semaglutide / Cyanocobalamin Injection is available via patient-specific prescription from our 503A compounding pharmacy, which means it is prepared specifically for individual patients based on a physicians determination that the medication is necessary to treat the patient. This personalized approach to medication preparation allows for adjustments in concentration, volume, or other characteristics to meet specific patient needs, as determined by the prescribing physician, that may not be addressed by commercially available products. The compounding process follows quality standards and regulatory requirements to ensure the potency and sterility of the final product. Compounded medications, such as Semaglutide/Cyanocobalamin, are not FDA-approved medications, and FDA does not review compounded medications for safety or efficacy.
The injection vehicle is carefully selected to ensure compatibility with both active ingredients while maintaining appropriate pH, osmolality, and other physical characteristics necessary for subcutaneous administration. Quality control measures are implemented throughout the compounding process to verify the accuracy of ingredient measurements, sterility of the final product, and absence of particulate matter or other contaminants.
The dosage and administration of Semaglutide / Cyanocobalamin Injection requires careful consideration of individual patient factors, treatment goals, and tolerance to ensure optimal therapeutic outcomes while minimizing adverse effects. The compounded formulation offers flexibility in dosing regimens, as determined necessary by the patient’s prescribing physician, with various concentrations available to accommodate different patient needs and treatment protocols. The medication is administered via subcutaneous injection, typically on a weekly basis, though dosing frequency and amounts may be individualized based on patient response and tolerability.
Initial dosing of Semaglutide / Cyanocobalamin Injection typically follows a gradual titration schedule designed to minimize gastrointestinal adverse effects while allowing patients to develop tolerance to the medication. The standard approach often begins with a low dose of semaglutide, commonly 0.25 mg weekly for the first four weeks, which serves primarily as an initiation dose to allow physiological adaptation rather than providing significant therapeutic effect. This conservative starting approach helps reduce the incidence and severity of nausea, vomiting, and other gastrointestinal symptoms that are most common during the early weeks of treatment.
Following the initial four-week period, the dose is typically increased to 0.5 mg of semaglutide weekly, which represents the first therapeutic dose level for many patients. This dose should be maintained for at least four weeks to assess tolerance and initial therapeutic response before considering further dose escalation. Some patients may achieve adequate glycemic control or weight loss at this dose level, particularly those with mild metabolic dysfunction or those who are particularly sensitive to the medication’s effects. The decision to maintain or increase the dose should be based on individual treatment goals, clinical response, and tolerance to adverse effects.
For patients requiring additional therapeutic effect, the dose may be increased to 1 mg of semaglutide weekly after at least four weeks at the 0.5 mg dose. This escalation should only occur if the lower dose is well-tolerated and additional benefit is needed to achieve treatment goals. Some patients may require an even higher dose of 2 mg weekly for optimal effect, particularly for weight management indications, though this should be approached cautiously with careful monitoring for adverse effects. The maximum recommended dose varies based on the specific indication and individual patient factors, but generally does not exceed 2.5 mg weekly for most patients.
The cyanocobalamin component in the formulation is provided at a consistent concentration of 0.5 mg/mL across all available strengths, delivering a substantial dose of vitamin B12 with each injection. This dosing far exceeds the recommended dietary allowance for vitamin B12 but is appropriate for injection therapy and ensures adequate B12 status even in patients with increased requirements or absorption issues. The high dose of cyanocobalamin is generally well-tolerated due to the vitamin’s water-soluble nature and efficient excretion of excess amounts.
Administration technique is crucial for optimal absorption and minimizing injection site reactions. The medication should be injected subcutaneously into the abdomen, thigh, or upper arm, with rotation of injection sites to prevent lipodystrophy or persistent local reactions. Patients should be instructed to clean the injection site with alcohol and allow it to dry before injection. The medication should be allowed to reach room temperature before administration if stored under refrigeration, as cold injections may be more painful and could potentially affect absorption kinetics.
The timing of injection relative to meals and other activities may influence tolerance and efficacy. While Semaglutide / Cyanocobalamin Injection can be administered at any time of day with or without meals, many patients find that consistent timing helps establish a routine and may influence the pattern of any adverse effects. Some patients prefer morning administration to allow any mild nausea to resolve during the day, while others may prefer evening injection if they experience fatigue or other symptoms following administration. The key is maintaining consistent weekly intervals between doses to ensure stable drug levels.
Missed dose management requires careful consideration to maintain therapeutic efficacy while avoiding adverse effects from irregular dosing. If a dose is missed, it should be administered as soon as remembered if within five days of the scheduled dose. If more than five days have passed, the missed dose should be skipped, and the next dose should be administered on the regularly scheduled day. Patients should be counseled never to administer extra doses to make up for missed doses, as this could increase the risk of adverse effects without providing additional therapeutic benefit.
Dose adjustments may be necessary based on various clinical scenarios and patient-specific factors. Patients experiencing persistent gastrointestinal adverse effects may benefit from slower titration schedules or maintaining lower doses for longer periods before attempting escalation. Some practitioners employ intermediate dose increases, such as escalating by 0.25 mg increments rather than doubling doses, to improve tolerance in sensitive patients. Conversely, patients with minimal adverse effects and inadequate therapeutic response may undergo more rapid titration under careful medical supervision.
Special populations may require modified dosing approaches for Semaglutide / Cyanocobalamin Injection. Elderly patients may be more sensitive to the medication’s effects and may require slower titration or lower maintenance doses, particularly if they have reduced renal function or are at higher risk for dehydration from gastrointestinal adverse effects. Patients with mild to moderate renal impairment generally do not require dose adjustment, but closer monitoring may be warranted, particularly for signs of gastrointestinal adverse effects that could lead to dehydration and worsening renal function.
The duration of therapy with Semaglutide / Cyanocobalamin Injection varies considerably based on treatment indication, response, and individual patient factors. For glycemic control in type 2 diabetes, treatment is typically continued indefinitely as long as it remains effective and well-tolerated, with periodic assessment of the ongoing need for therapy. For weight management, treatment duration may depend on achievement and maintenance of weight loss goals, with some patients requiring long-term therapy to maintain weight reduction while others may successfully transition to lifestyle modifications alone after achieving target weight.
Monitoring parameters during treatment should include regular assessment of therapeutic response, adverse effects, and relevant laboratory parameters. For patients with diabetes, regular monitoring of blood glucose levels and periodic measurement of hemoglobin A1c helps assess glycemic control and guide dose adjustments. Weight should be monitored regularly for all patients, along with vital signs including blood pressure and heart rate. Patients should be educated about signs and symptoms of potential serious adverse effects, including pancreatitis, gallbladder disease, and severe hypoglycemia if receiving concurrent antidiabetic therapy.
The transition between different GLP-1 receptor agonists or to and from Semaglutide / Cyanocobalamin Injection requires careful planning to maintain therapeutic effect while minimizing adverse effects. When switching from another weekly GLP-1 receptor agonist, the first dose of Semaglutide / Cyanocobalamin Injection can typically be administered at the time the next dose of the previous medication would have been due. When switching from a daily GLP-1 receptor agonist, treatment can usually begin the day after the last dose of the daily medication. Dose equivalency between different GLP-1 receptor agonists is not straightforward, and initial dosing should follow standard titration schedules regardless of the previous medication dose.
The use of Semaglutide / Cyanocobalamin Injection is contraindicated in several specific patient populations and clinical situations where the risks associated with treatment may outweigh potential benefits. Understanding these contraindications is essential for healthcare providers and patients to ensure safe and appropriate use of this compounded medication. The contraindications relate to both the semaglutide and cyanocobalamin components, and careful consideration must be given to each ingredient’s specific safety profile and potential risks.
A primary contraindication for Semaglutide / Cyanocobalamin Injection is a known hypersensitivity or allergic reaction to semaglutide, cyanocobalamin, or any of the excipients used in the formulation. Hypersensitivity reactions may manifest in various ways, ranging from local injection site reactions such as erythema, swelling, and pruritus to more severe systemic reactions including angioedema, anaphylaxis, or generalized urticaria. Patients who have experienced allergic reactions to other GLP-1 receptor agonists should be evaluated carefully before initiating treatment with this formulation, as cross-reactivity between different GLP-1 receptor agonists may occur in some individuals.
Personal or family history of medullary thyroid carcinoma represents another significant contraindication for the use of Semaglutide / Cyanocobalamin Injection. This contraindication stems from observations in rodent studies where semaglutide and other GLP-1 receptor agonists caused dose-dependent and treatment-duration-dependent thyroid C-cell tumors. Patients with Multiple Endocrine Neoplasia syndrome type 2, a genetic condition associated with increased risk of medullary thyroid carcinoma, should also avoid treatment with this medication.
The presence of diabetic retinopathy complications may require special consideration before initiating treatment with Semaglutide / Cyanocobalamin Injection. Some studies have suggested that rapid improvements in glycemic control with semaglutide may be associated with temporary worsening of diabetic retinopathy in certain patients, particularly those with pre-existing retinopathy. While this effect appears to be related to the magnitude and rapidity of glucose lowering rather than a direct effect of the medication itself, patients with a history of diabetic retinopathy should undergo careful ophthalmologic evaluation before and during treatment, and the potential risks should be weighed against the expected benefits of improved glycemic control.
Patients with a history of pancreatitis should be evaluated carefully before initiating treatment with Semaglutide / Cyanocobalamin Injection, as GLP-1 receptor agonists have been associated with acute pancreatitis in post-marketing surveillance, though a definitive causal relationship has not been established. The mechanism by which GLP-1 receptor agonists might contribute to pancreatitis risk remains unclear, but possibilities include effects on pancreatic enzyme secretion, changes in gallbladder motility leading to gallstone formation, or direct effects on pancreatic tissue. Patients with a history of chronic pancreatitis, pancreatic cancer, or other significant pancreatic disorders may not be suitable candidates for this therapy.
Severe gastrointestinal disease, particularly gastroparesis or other conditions causing significant delays in gastric emptying, may be exacerbated by treatment with Semaglutide / Cyanocobalamin Injection. Since semaglutide further slows gastric emptying as part of its mechanism of action, patients with pre-existing gastroparesis may experience worsening of symptoms including nausea, vomiting, bloating, and abdominal discomfort. Additionally, patients with inflammatory bowel disease, severe gastroesophageal reflux disease, or other significant gastrointestinal disorders should be monitored closely if treatment is initiated, as the gastrointestinal effects of semaglutide may worsen their underlying condition.
For the cyanocobalamin component, while generally considered safe with few absolute contraindications, certain conditions warrant caution. Leber’s hereditary optic neuropathy, a rare genetic disorder affecting the optic nerve, has been associated with rapid optic atrophy following cyanocobalamin administration in some cases. Patients with this condition or those with a family history suggestive of Leber’s disease should avoid cyanocobalamin-containing products or use alternative forms of vitamin B12 such as hydroxocobalamin under careful medical supervision.
Patients with severe renal impairment or end-stage renal disease requiring dialysis may need special consideration when using Semaglutide / Cyanocobalamin Injection. While semaglutide does not require dose adjustment for mild to moderate renal impairment, experience in patients with severe renal impairment is limited. The potential for gastrointestinal adverse effects, particularly nausea and vomiting, may lead to dehydration and worsening of renal function in vulnerable patients. Additionally, accumulation of cyanocobalamin metabolites may occur in severe renal impairment, though this is rarely clinically significant.
Hepatic impairment may also influence the suitability of Semaglutide / Cyanocobalamin Injection for certain patients. While semaglutide pharmacokinetics are not significantly altered in patients with hepatic impairment, the medication has not been extensively studied in patients with severe hepatic dysfunction. The metabolism and clearance of both components may be affected by significant liver disease, and patients with severe hepatic impairment should be monitored closely if treatment is deemed necessary.
Pregnancy represents a significant contraindication for Semaglutide / Cyanocobalamin Injection due to potential risks to fetal development. Animal reproduction studies with semaglutide have shown embryofetal toxicity, including structural abnormalities, at exposures below those expected at therapeutic doses in humans. Women of reproductive potential should be counseled about these risks and should use effective contraception during treatment. If pregnancy is planned, the medication should be discontinued at least two months before attempting conception due to the long washout period of semaglutide.
The use of Semaglutide / Cyanocobalamin Injection in pediatric populations has not been established, and the medication should not be used in patients under 18 years of age unless specifically justified by exceptional circumstances and under close specialist supervision. The effects of GLP-1 receptor agonists on growth and development in children and adolescents have not been fully characterized, and the long-term safety profile in pediatric populations remains unknown.
Patients with a history of suicidal ideation or attempts should be carefully evaluated before initiating treatment with Semaglutide / Cyanocobalamin Injection, as post-marketing reports have described occurrences of suicidal thoughts and behaviors in patients treated with GLP-1 receptor agonists. While a causal relationship has not been established, patients with depression or other psychiatric conditions should be monitored for changes in mood or behavior, particularly during the initial weeks of treatment or following dose adjustments.
The potential for drug interactions with Semaglutide / Cyanocobalamin Injection encompasses a broad range of medications, supplements, and even certain medical procedures, necessitating careful consideration of concurrent therapies and patient-specific factors. Understanding these interactions is crucial for optimizing therapeutic outcomes while minimizing the risk of adverse effects or reduced efficacy of either the compounded medication or concomitant treatments.
Semaglutide’s effect on gastric emptying represents one of the most significant mechanisms for potential drug interactions. By delaying gastric emptying, semaglutide may alter the rate and extent of absorption of orally administered medications, particularly those requiring rapid absorption for optimal effect or those with a narrow therapeutic window. Medications that are particularly susceptible to altered absorption kinetics include oral contraceptives, where delayed or reduced absorption could potentially compromise contraceptive efficacy. Women using oral contraceptives should be counseled about this potential interaction and may need to consider alternative or additional contraceptive methods, particularly during the initial weeks of treatment when gastric emptying effects may be most pronounced.
The interaction between Semaglutide / Cyanocobalamin Injection and other antidiabetic medications requires careful consideration to avoid hypoglycemia while achieving optimal glycemic control. When used concurrently with insulin or insulin secretagogues such as sulfonylureas or meglitinides, the risk of hypoglycemia may be increased, particularly during the initiation of therapy or following dose escalations. Healthcare providers often need to reduce the doses of these concurrent antidiabetic medications when starting semaglutide therapy, with subsequent adjustments based on glucose monitoring results and clinical response. The glucose-dependent nature of semaglutide’s insulin secretion generally results in a lower intrinsic risk of hypoglycemia compared to insulin or sulfonylureas, but the combination effect must be carefully managed.
Warfarin and other vitamin K antagonists may interact with Semaglutide / Cyanocobalamin Injection through multiple mechanisms. The altered gastric emptying and potential changes in food intake associated with semaglutide therapy may affect the absorption and metabolism of warfarin, potentially leading to fluctuations in international normalized ratio values. Additionally, if gastrointestinal adverse effects lead to reduced food intake or changes in dietary vitamin K consumption, warfarin dosing requirements may change. Patients receiving warfarin should undergo more frequent monitoring when initiating or adjusting semaglutide therapy, with warfarin dose adjustments made as necessary to maintain therapeutic anticoagulation.
The absorption of levothyroxine and other thyroid hormone preparations may be affected by concurrent use of Semaglutide / Cyanocobalamin Injection. Since levothyroxine requires an acidic gastric environment for optimal dissolution and absorption, and its absorption can be affected by the rate of gastric emptying, patients may experience variations in thyroid hormone levels when starting semaglutide therapy. Thyroid function tests should be monitored in patients receiving thyroid hormone replacement, and the timing of levothyroxine administration relative to semaglutide injection may need to be optimized to ensure consistent absorption.
Medications that affect gastrointestinal motility may have additive or antagonistic effects when used with Semaglutide / Cyanocobalamin Injection. Prokinetic agents such as metoclopramide or domperidone, which accelerate gastric emptying, may counteract some of the therapeutic effects of semaglutide on satiety and weight loss. Conversely, medications that further slow gastric emptying, such as anticholinergics or opioid analgesics, may exacerbate gastrointestinal adverse effects including nausea, vomiting, and constipation. The combination of multiple medications affecting gastrointestinal motility should be carefully evaluated, and patients should be monitored for both efficacy and tolerability.
The cyanocobalamin component of the injection may interact with certain medications that affect vitamin B12 absorption or metabolism. Metformin, commonly used in diabetes management, has been associated with reduced vitamin B12 absorption, potentially through effects on calcium-dependent vitamin B12-intrinsic factor absorption in the terminal ileum. While the injectable route of administration in Semaglutide / Cyanocobalamin Injection bypasses the need for gastrointestinal absorption, patients receiving high-dose or long-term metformin therapy may have increased B12 requirements that should be considered when determining appropriate dosing.
Proton pump inhibitors and H2 receptor antagonists, commonly used for gastroesophageal reflux disease and peptic ulcer disease, may affect vitamin B12 status through multiple mechanisms. These medications reduce gastric acid production, which is necessary for releasing dietary vitamin B12 from food proteins and for maintaining the stability of the B12-intrinsic factor complex. While this interaction primarily affects dietary B12 absorption rather than injected cyanocobalamin, patients on long-term acid suppression therapy may have depleted B12 stores that require consideration when initiating treatment.
Certain antibiotics may interact with the cyanocobalamin component through effects on vitamin B12 metabolism or efficacy. Chloramphenicol, though rarely used in modern practice, can antagonize the hematologic response to vitamin B12 in patients with megaloblastic anemia. Aminoglycosides, tetracyclines, and certain other antibiotics may alter gut microbiota composition, potentially affecting the small amount of vitamin B12 that is produced by colonic bacteria, though this is generally not clinically significant for patients receiving injectable B12 supplementation.
The timing of Semaglutide / Cyanocobalamin Injection relative to certain medical procedures requires consideration. For procedures requiring gastric emptying or precise timing of oral medication administration, such as upper endoscopy or surgery requiring general anesthesia, the delayed gastric emptying effect of semaglutide may need to be considered. Some guidelines recommend holding GLP-1 receptor agonists before procedures requiring sedation or general anesthesia to reduce the risk of aspiration, though specific recommendations may vary based on the procedure type and individual patient factors.
Interactions with supplements and herbal preparations should also be considered when using Semaglutide / Cyanocobalamin Injection. High-dose vitamin B12 supplements taken concurrently with the injection may lead to excessive B12 levels, though toxicity from vitamin B12 excess is extremely rare due to its water-soluble nature and efficient renal excretion. Herbal preparations that affect blood glucose levels, such as ginseng, fenugreek, or bitter melon, may have additive glucose-lowering effects when combined with semaglutide, potentially increasing the risk of hypoglycemia particularly in patients receiving other antidiabetic medications.
The consumption of alcohol while using Semaglutide / Cyanocobalamin Injection may increase the risk of hypoglycemia, particularly in patients with diabetes who are also receiving insulin or sulfonylureas. Alcohol can impair hepatic gluconeogenesis and may mask the symptoms of hypoglycemia, making detection and treatment more challenging. Additionally, excessive alcohol consumption can affect vitamin B12 absorption and metabolism, potentially reducing the efficacy of the cyanocobalamin component. Patients should be counseled about moderate alcohol consumption and the importance of monitoring blood glucose levels more carefully when drinking alcohol.
The side effect profile of Semaglutide / Cyanocobalamin Injection encompasses a range of potential adverse reactions that vary in frequency, severity, and clinical significance. Understanding these potential side effects is essential for informed decision-making by healthcare providers and patients, as well as for appropriate monitoring and management strategies during treatment. The adverse effects associated with this compounded medication primarily reflect those observed with semaglutide, as cyanocobalamin is generally well-tolerated with minimal side effects when administered at therapeutic doses.
Gastrointestinal adverse effects represent the most commonly observed side effects of Semaglutide / Cyanocobalamin Injection, occurring in a substantial proportion of patients, particularly during the initial weeks of treatment or following dose escalations. Nausea is the most frequently reported adverse effect, potentially affecting thirty to forty percent of patients at some point during treatment, though the intensity typically diminishes over time as tolerance develops. The nausea associated with semaglutide is thought to result from both its effects on gastric emptying and its action on central nervous system centers involved in nausea and appetite regulation. Most patients experience mild to moderate nausea that can be managed through dietary modifications, such as eating smaller, more frequent meals and avoiding high-fat or spicy foods that may exacerbate symptoms.
Vomiting occurs less frequently than nausea but may affect fifteen to twenty percent of patients, particularly those who experience severe nausea or those who do not implement dietary modifications to manage gastrointestinal symptoms. Diarrhea and constipation are also commonly observed, each potentially affecting ten to fifteen percent of patients, with individual susceptibility varying based on factors such as baseline gastrointestinal function, dietary habits, and concurrent medications. The mechanism underlying these opposing gastrointestinal effects likely relates to semaglutide’s complex effects on gastrointestinal motility, with some patients experiencing accelerated intestinal transit while others experience overall slowing of gastrointestinal motility.
Abdominal pain, discomfort, or bloating may occur in approximately ten percent of patients receiving Semaglutide / Cyanocobalamin Injection. These symptoms often correlate with other gastrointestinal effects and may be particularly noticeable after meals due to delayed gastric emptying. Dyspepsia, characterized by upper abdominal discomfort, early satiety, or postprandial fullness, may affect a similar proportion of patients and can contribute to reduced caloric intake and weight loss, though it may also impact quality of life if severe or persistent.
Injection site reactions represent another category of commonly observed side effects, though these are typically mild and self-limiting. Local reactions may include erythema, pruritus, induration, or mild pain at the injection site, potentially affecting five to ten percent of patients. These reactions usually resolve within a few days and rarely require treatment discontinuation. Proper injection technique, including rotation of injection sites and allowing the medication to reach room temperature before administration, may help minimize these local reactions.
Hypoglycemia risk with Semaglutide / Cyanocobalamin Injection varies considerably depending on concurrent antidiabetic therapy. When used as monotherapy or in combination with metformin, the risk of clinically significant hypoglycemia is relatively low, typically less than five percent, due to the glucose-dependent nature of semaglutide’s insulin secretion. However, when combined with insulin or sulfonylureas, the risk of hypoglycemia increases substantially, potentially affecting twenty to thirty percent of patients if doses of concurrent medications are not appropriately adjusted. Symptoms of hypoglycemia may include tremor, palpitations, anxiety, sweating, hunger, and confusion, progressing to more severe neurological symptoms if untreated.
Cardiovascular side effects, while less common, may include changes in heart rate, with some patients experiencing a mild increase in resting heart rate of two to three beats per minute on average. This effect is thought to be mediated through GLP-1 receptors in the cardiovascular system and is generally not clinically significant, though patients with certain arrhythmias or those sensitive to heart rate changes should be monitored. Some patients may experience transient changes in blood pressure, though long-term cardiovascular outcomes studies have generally shown beneficial or neutral effects of GLP-1 receptor agonists on cardiovascular events.
Rare but potentially serious adverse effects require careful consideration and monitoring. Pancreatitis, though uncommon with an incidence of less than one percent, represents a potentially serious complication that requires immediate medical attention. Symptoms suggestive of pancreatitis include severe, persistent abdominal pain that may radiate to the back, often accompanied by nausea and vomiting. The mechanism linking GLP-1 receptor agonists to pancreatitis remains unclear, and causality has not been definitively established, but vigilance for symptoms is warranted.
Gallbladder-related adverse events, including cholelithiasis and cholecystitis, have been reported more frequently in patients receiving semaglutide compared to placebo in clinical trials, with an incidence of approximately two to three percent. The increased risk may relate to rapid weight loss, changes in gallbladder motility, or alterations in bile acid composition. Patients experiencing right upper quadrant pain, particularly if accompanied by fever or jaundice, should be evaluated for gallbladder disease.
Diabetic retinopathy complications have been observed in some patients with pre-existing retinopathy who experience rapid improvements in glycemic control with semaglutide therapy. This phenomenon, sometimes referred to as early worsening of diabetic retinopathy, appears to be related to the magnitude and speed of glucose lowering rather than a direct effect of the medication. The incidence is relatively low, affecting less than five percent of patients with pre-existing retinopathy, but regular ophthalmologic monitoring is recommended for patients with diabetes-related eye disease.
Renal effects, including acute kidney injury, have been reported rarely in post-marketing surveillance, typically in the context of severe gastrointestinal adverse effects leading to dehydration. Patients experiencing persistent vomiting or diarrhea should be monitored for signs of dehydration and renal dysfunction, with appropriate fluid replacement and potentially temporary discontinuation of the medication if necessary. Changes in renal function may also occur in patients experiencing significant weight loss, as glomerular hyperfiltration associated with obesity improves.
Allergic reactions to Semaglutide / Cyanocobalamin Injection, while rare, may range from mild local reactions to severe systemic responses. Urticaria, pruritus without rash, and angioedema have been reported in less than one percent of patients. Anaphylactic reactions are extremely rare but have been reported with GLP-1 receptor agonists. Patients experiencing signs of allergic reactions should discontinue the medication and seek appropriate medical attention.
The cyanocobalamin component of the injection is generally associated with minimal side effects when administered at therapeutic doses. Rare adverse effects specific to cyanocobalamin may include mild transient diarrhea, particularly with initial doses, and very rarely, allergic reactions in sensitive individuals. Some patients may experience a transient increase in acne or rosacea-like skin reactions, thought to be related to the effects of vitamin B12 on skin bacteria, though this is uncommon and typically mild.
Neuropsychiatric effects have been reported in post-marketing surveillance of GLP-1 receptor agonists, though the incidence and causal relationship remain unclear. Some patients may experience mood changes, including depression or anxiety, particularly during the initial weeks of treatment. Sleep disturbances, including insomnia or vivid dreams, have been reported by a small percentage of patients. Headache is reported by approximately five to ten percent of patients, though this often improves with continued treatment.
Laboratory abnormalities may be observed in some patients receiving Semaglutide / Cyanocobalamin Injection. Elevations in lipase and amylase levels are commonly seen, often without clinical signs of pancreatitis, making interpretation challenging. Increases in calcitonin levels have been observed in some patients, though the clinical significance remains uncertain. Changes in thyroid function tests are generally not observed, despite theoretical concerns based on animal studies showing thyroid C-cell effects.
If pregnancy occurs while using this medication, you should immediately contact your healthcare provider to discuss whether you should continue using this compounded medication.
You should follow your healthcare providers’ instructions regarding when this medication can be resumed, if discontinued, and whether this medication may be used during breastfeeding.
Proper storage of Semaglutide / Cyanocobalamin Injection is essential for maintaining medication stability and potency throughout its designated beyond-use date. The storage requirements for this compounded medication reflect the need to preserve both the semaglutide and cyanocobalamin components while preventing degradation, contamination, or other changes that could negatively affect the medication. . Understanding and following appropriate storage guidelines ensures that patients receive the full therapeutic benefit of the medication while minimizing the risk of using degraded or contaminated product.
The primary storage requirement for Semaglutide / Cyanocobalamin Injection is maintenance of appropriate temperature conditions. The medication should typically be stored under refrigeration at temperatures between 36°F and 46°F (2°C to 8°C) to maintain optimal stability of both active ingredients. Refrigeration helps prevent degradation of the peptide structure of semaglutide and maintains the stability of cyanocobalamin, which can be sensitive to heat and light exposure. The medication should be stored in the main compartment of the refrigerator rather than in the door, where temperature fluctuations are more common due to frequent opening and closing.
While refrigeration is generally preferred for long-term storage, Semaglutide / Cyanocobalamin Injection may be stable at room temperature for limited periods, which can be important for patient convenience and compliance. This room temperature stability allows patients to travel with their medication or keep it readily accessible without constant refrigeration, though the total time at room temperature should not exceed the specified limits.
Protection from light is another critical storage consideration for Semaglutide / Cyanocobalamin Injection, particularly due to the photosensitivity of cyanocobalamin. The medication should be stored in its original packaging or container, which is typically designed to provide adequate light protection. Exposure to direct sunlight or bright artificial light should be avoided, as this can lead to degradation of the cyanocobalamin component and potentially affect the semaglutide as well. If the medication must be removed from its original packaging, it should be kept in a light-protected area such as a closed drawer or cabinet.
Freezing of Semaglutide / Cyanocobalamin Injection must be avoided, as this can cause irreversible damage to the medication’s structure and potentially negatively affect the medication. Freezing can cause protein aggregation in the semaglutide component and may damage the physical structure of the solution, potentially leading to particulate formation or changes in drug concentration. If the medication has been frozen, even briefly, it should not be used and should be properly disposed of according to pharmacy guidelines. Patients should be counseled to store the medication away from the freezer compartment and to be aware of areas in their refrigerator that may be prone to freezing.
The medication should be protected from excessive heat and humidity, which can accelerate degradation and potentially support microbial growth. Storage areas near heat sources such as stoves, radiators, or heating vents should be avoided, as should storage in bathrooms where humidity levels may be high due to bathing and showering activities. The medication should never be left in vehicles, particularly during warm weather, where temperatures can rapidly exceed safe storage limits and cause degradation of the active ingredients.
Proper handling during storage is essential to maintain medication integrity. The vial should be kept upright when possible and should not be shaken vigorously, as this could potentially cause foaming or degradation of the semaglutide peptide structure. Gentle mixing by rolling the vial between the hands may be appropriate if settling has occurred, but aggressive agitation should be avoided. The rubber stopper should not be removed from the vial, as this compromises sterility and could lead to contamination.
The beyond-use date assigned to Semaglutide / Cyanocobalamin Injection by the compounding pharmacy must be strictly observed. This date is determined based on stability studies, compounding conditions, and regulatory guidelines, typically ranging from 90 days from the date of compounding depending on storage conditions and specific formulation factors. The beyond-use date differs from an expiration date in that it represents the last date the compounded preparation should be used, taking into account the specific conditions of preparation and storage.
Patients should be instructed to check the medication before each use for any signs of degradation or contamination. The solution should be light red, without visible particles, cloudiness, or discoloration. Any change in appearance, including crystallization, precipitation, or color change, indicates that the medication should not be used and should be returned to the pharmacy for proper disposal. The presence of particles or cloudiness could indicate contamination or degradation and poses a safety risk if injected.
Multi-dose vial considerations require additional attention to storage and handling practices to maintain sterility throughout the use period. Once the vial is first punctured, the risk of contamination increases, and additional precautions may be necessary. The date of first use should be recorded on the vial, and the medication should be discarded after the specified period following initial puncture, typically 28 days, even if medication remains and the beyond-use date has not been reached. Between uses, the vial septum should be protected from contamination, and proper aseptic technique should be used for each withdrawal.
Transportation and temporary storage outside the home require special consideration to maintain appropriate storage conditions. When traveling, the medication should be transported in an insulated container with ice packs if refrigeration will not be available, taking care to prevent direct contact between the medication and ice to avoid freezing. For air travel, the medication should be carried in hand luggage rather than checked baggage to maintain temperature control and prevent loss. Patients should be provided with a letter from their healthcare provider or pharmacy documenting the medical necessity of carrying injection supplies.
Disposal of unused or expired Semaglutide / Cyanocobalamin Injection should follow appropriate guidelines to prevent environmental contamination and accidental exposure. The medication should not be disposed of in household trash or flushed down toilets or drains, as this can lead to environmental contamination and potential exposure of others to the medication. Many communities have pharmaceutical take-back programs or events where medications can be safely disposed of, and some pharmacies offer disposal services for medications they have dispensed. If no take-back options are available, the FDA provides guidelines for home disposal that involve mixing the medication with unpalatable substances and sealing in a container before disposal.
Emergency storage situations, such as power outages affecting refrigeration, require prompt action to maintain medication integrity. If refrigeration is temporarily unavailable, the medication should be kept in the coolest available location, avoiding direct sunlight and heat sources. Insulated containers with ice packs can provide temporary refrigeration, but care must be taken to prevent freezing. The duration of storage outside recommended temperature ranges should be documented, and the pharmacy should be consulted about whether the medication remains safe to use based on the specific conditions and duration of improper storage.
What exactly is Semaglutide / Cyanocobalamin Injection and how does it differ from regular semaglutide medications?
Semaglutide / Cyanocobalamin Injection is a compounded medication that combines semaglutide, a GLP-1 receptor agonist, with cyanocobalamin (vitamin B12) in a single injectable formulation. Compounded medications are prepared for specific patients based on physician determinations of patient need. Compounded medications provide for customization of strengths and volumes, as determined necessary by the prescribing physician for the identified patient, that may not be available in commercial products. Compounded medications are not FDA-approved drugs. FDA does not review compounded medications for safety or efficacy.
Can Semaglutide / Cyanocobalamin Injection be used with other diabetes medications?
Semaglutide / Cyanocobalamin Injection can potentially be used in combination with various other diabetes medications, though careful consideration and monitoring are essential to ensure safety and optimize therapeutic outcomes. The medication is commonly used alongside metformin, with the combination often providing complementary mechanisms of action for improved glycemic control. When combined with insulin or sulfonylureas, dose adjustments of these medications are frequently necessary to reduce the risk of hypoglycemia, as the glucose-lowering effects may be additive. SGLT-2 inhibitors and thiazolidinediones may also be used concurrently, though patients should be monitored for any unexpected interactions or adverse effects. The key principle is that any combination therapy should be initiated and monitored under close medical supervision, with dose adjustments made based on glucose monitoring results and individual patient response.
What should I do if I experience persistent nausea or other gastrointestinal side effects?
Managing gastrointestinal side effects, particularly nausea, requires a multifaceted approach that may include both behavioral modifications and potential medical interventions. Dietary adjustments often provide significant relief, including eating smaller, more frequent meals rather than large portions, avoiding high-fat or spicy foods that may exacerbate symptoms, and eating slowly to allow for better digestion. Staying well-hydrated is crucial, particularly if experiencing vomiting or diarrhea, though large volumes of fluid with meals should be avoided as this may worsen symptoms. Some patients find relief by taking the injection at a different time of day or by ensuring the medication has reached room temperature before injection. If symptoms persist despite these measures, healthcare providers may consider slower dose titration schedules, temporary dose reductions, or prescribing anti-nausea medications for short-term relief. Persistent severe symptoms that interfere with daily activities or lead to dehydration should prompt immediate medical consultation, as medication discontinuation or alternative treatments may be necessary.
Is it safe to consume alcohol while using Semaglutide / Cyanocobalamin Injection?
Alcohol consumption while using Semaglutide / Cyanocobalamin Injection requires careful consideration and moderation due to several potential interactions and risks. Alcohol can increase the risk of hypoglycemia, particularly in patients with diabetes who are also taking insulin or sulfonylureas in combination with semaglutide. The symptoms of hypoglycemia may be masked by alcohol intoxication, making detection and treatment more challenging. Additionally, alcohol can exacerbate gastrointestinal side effects such as nausea and may contribute to dehydration if combined with vomiting or diarrhea caused by the medication. Moderate alcohol consumption, defined as up to one drink per day for women and up to two drinks per day for men, is generally considered acceptable for most patients, though individual tolerance and circumstances should be considered. Patients should be advised to monitor blood glucose levels more carefully when consuming alcohol and to ensure adequate food intake to prevent hypoglycemia.
How should I handle travel when I need to take Semaglutide / Cyanocobalamin Injection with me?
Traveling with Semaglutide / Cyanocobalamin Injection requires advance planning to ensure proper storage and compliance with transportation regulations. For air travel, the medication and injection supplies should be packed in carry-on luggage to maintain temperature control and prevent loss, with a letter from the healthcare provider documenting medical necessity. The medication should be transported in an insulated cooler with ice packs if refrigeration will not be available during travel, taking care to prevent direct contact with ice to avoid freezing. For extended trips, research should be conducted on refrigeration availability at the destination, and consideration should be given to bringing extra supplies in case of travel delays. Time zone changes may affect the weekly dosing schedule, and patients should plan how to maintain consistent seven-day intervals between doses while accommodating their travel schedule. International travel may require additional documentation and research into local regulations regarding traveling with injectable medications.
What happens if I miss a dose of Semaglutide / Cyanocobalamin Injection?
Managing missed doses appropriately is important for maintaining therapeutic benefits while avoiding adverse effects from irregular dosing patterns. If a dose is missed and remembered within five days of the scheduled injection day, the dose should be administered as soon as possible, and the regular weekly schedule should be resumed from that new day. If more than five days have passed since the missed dose, that dose should be skipped entirely, and the next dose should be taken on the originally scheduled day to maintain the weekly interval. Patients should never double up on doses or take extra medication to compensate for missed doses, as this increases the risk of adverse effects without providing additional therapeutic benefit. Frequent missed doses may compromise treatment effectiveness, and patients experiencing difficulty maintaining their dosing schedule should discuss strategies with their healthcare provider, such as setting reminders or adjusting the injection day to better fit their routine.
Can Semaglutide / Cyanocobalamin Injection cause thyroid problems or cancer?
The potential relationship between GLP-1 receptor agonists like semaglutide and thyroid C-cell tumors has been observed in rodent studies, where dose-dependent and duration-dependent increases in thyroid C-cell tumors were noted. However, the relevance of these findings to humans remains uncertain, as human thyroid C-cells have much lower expression of GLP-1 receptors compared to rodents. As a precautionary measure, the medication is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or Multiple Endocrine Neoplasia syndrome type 2. Routine monitoring of thyroid function tests is not typically required unless clinical symptoms suggest thyroid dysfunction. Patients should be educated about potential symptoms of thyroid tumors, including persistent hoarseness, difficulty swallowing, or a neck mass, though these symptoms are rare and may have many other causes. The overall risk appears to be very low, but the theoretical concern necessitates appropriate patient selection and informed consent.
Will I need to take Semaglutide / Cyanocobalamin Injection forever?
The duration of treatment with Semaglutide / Cyanocobalamin Injection varies considerably based on individual circumstances, treatment goals, and ongoing response to therapy. For type 2 diabetes management, the medication may be needed long-term as diabetes is a chronic condition requiring ongoing treatment, though some patients may be able to reduce or discontinue medication if significant weight loss and lifestyle modifications lead to remission of their diabetes. For weight management, some patients may use the medication until achieving their goal weight and then attempt to maintain weight loss through lifestyle modifications alone, while others may require continued treatment to prevent weight regain. The vitamin B12 component provides ongoing supplementation that may be beneficial for many patients, particularly those with risk factors for deficiency. Regular reassessment with healthcare providers helps determine the ongoing need for treatment, with consideration of factors such as treatment response, side effects, and changes in health status or treatment goals.
What should I know about the injection technique and rotating injection sites?
Proper injection technique is crucial for optimal medication absorption and minimizing injection site reactions. The medication should be injected subcutaneously into fatty tissue of the abdomen, thigh, or upper arm, with the abdomen often being preferred due to consistent absorption and ease of access. Injection sites should be rotated with each weekly dose to prevent lipodystrophy, scarring, or persistent irritation at any single location. The injection site should be cleaned with alcohol and allowed to dry completely before injection to prevent stinging and ensure proper sterilization. The needle should be inserted at a 90-degree angle for most patients, though very thin individuals may need to use a 45-degree angle or pinch the skin to ensure subcutaneous rather than intramuscular injection. After injection, slight pressure may be applied with a cotton ball or gauze, but the area should not be massaged as this may affect absorption. Patients should be trained on proper disposal of used needles in a sharps container and should never reuse needles due to infection risk and potential for needle damage that could cause pain or tissue trauma.
Administration Instructions
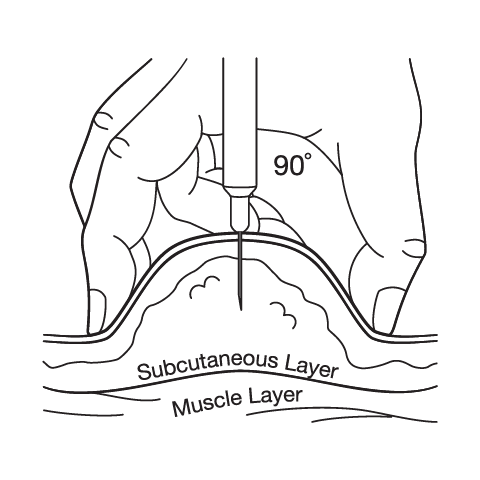
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Tirzepatide / Niacinamide Injection
Tirzepatide / Niacinamide Injection Tirzepatide ODT
Tirzepatide ODT Sermorelin Acetate Injection
Sermorelin Acetate Injection Bella Capsules
Bella Capsules Lipo-C Injection
Lipo-C Injection Lipo-B Injection
Lipo-B Injection Phentermine HCl Capsules
Phentermine HCl Capsules Lipo Injection
Lipo Injection Sermorelin Acetate ODT
Sermorelin Acetate ODT Ondansetron ODT
Ondansetron ODT