Product Overview
Quad-Mix Injection is a compounded intracavernosal therapy for erectile dysfunction (ED) combining papaverine HCl, phentolamine mesylate, prostaglandin E1 (alprostadil), and atropine sulfate in a single sterile formulation.
It is prescribed when oral phosphodiesterase-5 inhibitors fail or are contraindicated, offering a potent second-line option that leverages four complementary pharmacologic pathways to improve penile blood inflow and veno-occlusion.[1]
The drug mixture is prepared individually by a 503A compounding pharmacy rather than manufactured commercially, so it has not undergone FDA pre-market review; instead, its safety and efficacy derive from decades of urologic experience with each component and with multidrug injection protocols.[2]
Quad-Mix may achieve stronger and more reliable erections than single-agent injections because papaverine increases cyclic nucleotide levels, phentolamine blocks sympathetic vasoconstriction, alprostadil directly raises cAMP in cavernosal smooth muscle, and atropine removes cholinergic inhibition.[3]
Clinical studies report high success rates in men with severe vasculogenic or diabetes-related ED who previously failed oral medication, underscoring the synergy of the four-drug combination.[4]
Lyophilized vials are reconstituted to yield either 30 mg papaverine / 2 mg phentolamine / 20 µg PGE1 / 200 µg atropine per mL (standard) or double those latter three concentrations in “Super” Quad-Mix.[21]
Initial in-office titration typically begins with 0.05 mL, advancing cautiously to the lowest volume that produces a satisfactory erection; self-injection employs a 29-31 gauge needle into the lateral shaft.[22]
Maximum use is three times weekly with ≥ 24 h between doses, never exceeding the physician-specified volume.[23]
An effective dose yields an erection within 5-15 min lasting ≤ 1 h; erections approaching 2 h signal the need for dose reduction.[24]
Overview: Quad-Mix induces erection by simultaneously relaxing arterial and trabecular smooth muscle, dilating cavernosal arteries, and compressing emissary veins to trap blood.[5]
Papaverine: As a non-selective phosphodiesterase inhibitor, papaverine raises intracellular cAMP and cGMP, producing potent vasodilation within corpus cavernosum tissue.[6]
Phentolamine: This non-selective α-adrenergic antagonist blocks norepinephrine-mediated vasoconstriction, eliminating sympathetic tone that maintains the penis in a flaccid state and enhancing arterial inflow.[7]
Prostaglandin E1 (alprostadil): Binding EP receptors activates adenylate cyclase, further elevating cAMP and directly relaxing cavernosal smooth muscle while aiding venous occlusion.[8]
Atropine: Low-dose antimuscarinic action suppresses inhibitory cholinergic input, facilitating nitric-oxide-mediated relaxation and improving the rigidity and duration of erections in non-responders to tri-drug mixes.[9]
Synergy: By engaging cyclic nucleotide elevation, adrenergic blockades, prostaglandin signaling, and anticholinergic modulation in concert, Quad-Mix maximizes erectile response even when one pathway is compromised.[10]
Quad-Mix is contraindicated in patients with hypersensitivity to any component, disorders predisposing to priapism (sickle-cell disease, leukemia, multiple myeloma), significant penile anatomical deformities or implants, or medical advice against sexual activity (e.g., unstable cardiovascular disease).[11]
Individuals on nitrate therapy must not use intracavernosal agents.[12]
Concurrent use with oral PDE-5 inhibitors or other intracavernosal drugs may potentiate hypotension and markedly increase priapism risk, so only one ED modality should be used at a time.[13]
Systemic vasodilators, excessive alcohol, or potent antihypertensives can augment hypotensive effects, while anticoagulant therapy can heighten injection-site bleeding; clinicians balance benefits against these considerations.[14]
Common local reactions include transient penile pain, minor bleeding, bruising, or burning during erection; these are usually mild and improve with proper technique.[15]
Serious adverse events, though uncommon, comprise priapism (> 4 h) requiring emergency care and penile fibrosis from chronic misuse; strict dose titration and limited injection frequency mitigate these risks.[16]
Occasional systemic effects-dizziness, flushing, tachycardia-stem from minimal systemic absorption and typically resolve spontaneously.[17]
Quad-Mix is not indicated for women. Men whose partners are pregnant should use condoms because seminal prostaglandin E1 may theoretically stimulate uterine activity.[18]
No evidence suggests the therapy affects male fertility, but it offers no contraception; partners desiring to avoid pregnancy must use appropriate methods.[19]
Overall, Quad-Mix’s pregnancy considerations center on protecting a pregnant partner, not the male user.[20]
Unmixed vials remain stable at 20-25 °C, protected from moisture and light.[25]
After reconstitution, solution must be refrigerated at 2-8 °C and discarded at the beyond-use date assigned by the pharmacy; freezing is prohibited.[26]
During travel, patients transport reconstituted vials in insulated containers or carry unmixed powder plus diluent to reconstitute onsite, maintaining sterility throughout.[27]
- Montague, D. K., Jarow, J. P., Broderick, G. A., et al. (2005). The management of erectile dysfunction: An AUA update. Journal of Urology, 174(1), 230-239.
- U.S. Food and Drug Administration. (2018). Compounded drugs are not FDA-approved. https://www.fda.gov/drugs/human-drug-compounding
- Andersson, K. E. (2001). Pharmacology of penile erection. Pharmacological Reviews, 53(3), 417-450.
- Montorsi, F., Guazzoni, G., Bergamaschi, F., et al. (1993). Effectiveness and safety of multidrug intracavernous therapy for vasculogenic impotence. Urology, 42(5), 554-558.
- Traish, A. M., Park, K., Li, W., Kim, S. W., & Lysiak, J. J. (1998). Phentolamine mesylate relaxes penile corpus cavernosum tissue by adrenergic and non-adrenergic mechanisms. International Journal of Impotence Research, 10(4), 215-223.
- Porst, H. (1996). The rationale for prostaglandin E₁ in erectile failure: A survey of worldwide experience. Journal of Urology, 155(3), 802-815.
- Bank, A. J., Babcock, D., & Sigurjonsson, A. (1992). Systemic haemodynamic effects of intracavernosal papaverine and phentolamine. American Journal of Cardiology, 70(22), 1433-1437.
- Lue, T. F. (2000). Erectile dysfunction. New England Journal of Medicine, 342(24), 1802-1813. https://doi.org/10.1056/NEJM200006153422407
- Fathy, H., Arafa, M., & Abdel-Hamid, I. A. (2007). Atropine-enhanced intracavernosal therapy for erectile dysfunction: A randomized study. Journal of Sexual Medicine, 4(4 Pt 1), 944-951.
- Brant, W. O., Bella, A. J., Lentz, A. C., & Brock, G. B. (2014). Intracavernosal injection for erectile dysfunction: Patient preferences and outcomes. Translational Andrology and Urology, 3(1), 2-8.
- Medscape. (2023). Alprostadil (Caverject) - contraindications & precautions. Medscape Drugs & Diseases. https://reference.medscape.com/drug/caverject
- American Urological Association. (2018). Guideline on erectile dysfunction. https://www.auanet.org/guidelines
- Drugs..com. (n.d.). Interaction report: Papaverine and sildenafil. Drugs.com. Retrieved June 9 2025 from https://www.drugs.com/interactions
- Kostis, J. B., & Dobrzynski, J. M. (1998). Alpha-blockers for benign prostatic hyperplasia and potential risk of hypotension. Drugs, 55(2), 237-241.
- McMahon, C. G. (1993). Penile pain associated with intracavernosal alprostadil therapy: Incidence and management. Australian and New Zealand Journal of Medicine, 23(6), 716-721.
- Carson, C. C. (1996). Priapism associated with intracavernosal injection therapy. Journal of Urology, 156(2), 582-587.
- Levine, L. A., Dimitriou, R. J., & Fakouri, B. (2011). Side-effects and complications of intracavernosal therapy for erectile dysfunction. Journal of Sexual Medicine, 8(6), 1764-1772.
- Drugs..com. (2024). Alprostadil use during pregnancy. Drugs.com. https://www.drugs.com/pregnancy/alprostadil.html
- National Institutes of Health. (2023). Erectile dysfunction fact sheet. https://www.nichd.nih.gov/health/topics/erectile
- European Association of Urology. (2024). EAU guidelines on male sexual and reproductive health: Erectile dysfunction. https://uroweb.org/guidelines
- StatPearls Publishing. (2025). Alprostadil intrapenile injection. In StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470413
- Mulhall, J. P., Guhring, P., Parker, M., Hopps, C., & Boorjian, S. A. (1995). An office-based diagnostic and therapeutic algorithm for erectile dysfunction. International Journal of Impotence Research, 7(3), 151-157.
- Grober, E. D., Domes, T., Premsagar, I. C., et al. (2012). Long-term outcomes of penile injection therapy. Canadian Urological Association Journal, 6(5), 380-385.
- Hellstrom, W. J. G., & Bivalacqua, T. J. (2000). Pharmacologic erection enhancement: Efficacy and safety. Current Urology Reports, 1(1), 80-86.
- United States Pharmacopeia. (2022). Handling and transport of sterile compounded preparations. In USP Compounding Compendium.
- Shotts, S. D., Reiter, R. E., & Levine, L. A. (2008). Stability of alprostadil in compounded erectile-dysfunction formulations. International Journal of Pharmaceutical Compounding, 12(4), 324-330.
- Newton, D. W. (2012). Stability of papaverine hydrochloride in sterile compounded injections. Hospital Pharmacy, 47(6), 457-462.
- Bechara, A. J., Romano, S., & Mogas-Saavedra, M. (2006). Quadruple-drug intracavernosal therapy for refractory erectile dysfunction. Journal of Sexual Medicine, 3(Suppl 1), 48-54.
- Rosen, R. C., Padma-Nathan, H., & Shabsigh, R. (2004). Time to onset of erection after intracavernosal injection therapy. International Journal of Impotence Research, 16(6), 492-495.
- Ralph, D. J., Christie, W., & Pryor, J. P. (1998). Intracavernosal injection therapy: Patient acceptability and long-term compliance. BJU International, 81(6), 699-703.
- Padma-Nathan, H. (1993). Incidence, severity and management of penile pain with intracavernosal prostaglandin E₁. Journal of Urology, 149(2), 302-305.
- Khan, M. A., Muneer, A., & Ralph, D. J. (1999). Long-term complications of intracavernosal injection therapy. International Journal of Impotence Research, 11(4), 205-209.
- Hatzimouratidis, K., Hatzichristou, D., & Burnett, A. L. (2010). Strategies for managing phosphodiesterase-5 inhibitor non-responders. International Journal of Impotence Research, 22(2), 87-97.
- Bettocchi, C., Palumbo, F., Spilotros, M., et al. (2019). Penile prosthesis implants: Patient and partner satisfaction. Journal of Sexual Medicine, 16(8), 1236-1243.
- Goldberg, R. A., Schmidt, C., & Klausner, A. P. (2006). Combination therapy with phosphodiesterase inhibitors and intracavernosal injections: A case series. Urology, 67(6), 1176-1180.
- Centers for Disease Control and Prevention. (2018). Safe medication disposal guidelines. https://www.cdc.gov/medicationsafety/disposal
- U.S. Food and Drug Administration. (2020). Compounded drug products: Questions and answers. https://www.fda.gov/drugs/human-drug-compounding/compounded-drug-products-questions-and-answers
How does Quad-Mix differ from Tri-Mix?
Quad-Mix adds atropine, giving a fourth mechanism that benefits men who were partial or non-responders to Tri-Mix.[28]
When will the erection start and how long will it last?
Typically within 5-15 min, subsiding by 60 min when dosed correctly; monitor and seek help if rigidity persists past 4 h.[29]
How do I inject it?
A fine needle delivers the prescribed volume into the lateral penile shaft; clinicians demonstrate and confirm proper self-injection before home use.[30]
Does it hurt?
Most men describe only a brief pinch; mild post-injection ache from alprostadil is common but tolerable.[31]
What side effects need urgent care?
Priapism > 4 h warrants emergency treatment; new penile nodules or curvature require physician evaluation.[32]
Can I use Quad-Mix if Sildenafil or Tadalafil failed?
Yes; multidrug injections succeed in many PDE-5-inhibitor non-responders due to their direct cavernosal action.[33]
What if it still doesn’t work?
Technique review, formulation adjustment, vacuum devices, or penile prosthesis surgery may be considered.[34]
May I combine it with pills?
Combination is generally unsafe; use only one ED therapy at a time unless expressly directed by a specialist.[35]
How should I store it?
Powder at room temperature, reconstituted solution refrigerated; discard after the beyond-use date.[36]
Is Quad-Mix FDA-approved?
No; it is compounded under section 503A for an individual prescription, so quality relies on pharmacy standards and clinician oversight.[37]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
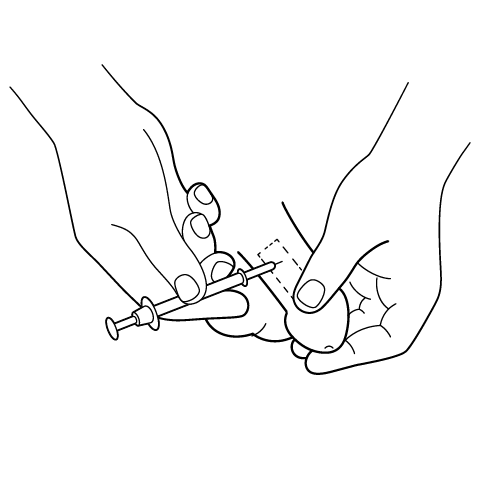
Penile Injection Instructions
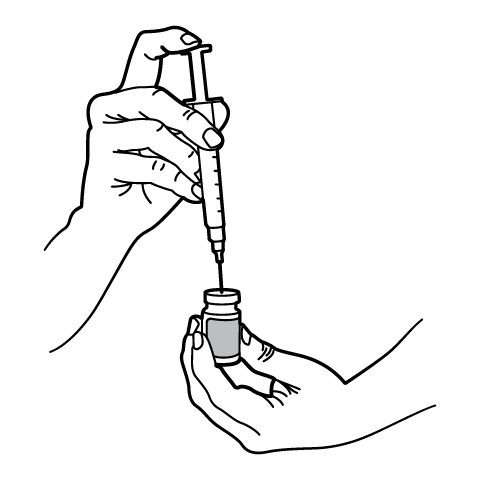
Reconstitution Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.



 Bi-Mix Injection
Bi-Mix Injection Tri-Mix Injection
Tri-Mix Injection Phenylephrine HCl Injection
Phenylephrine HCl Injection Vardenafil Troches
Vardenafil Troches Tadalafil Troches
Tadalafil Troches Tadalafil ODT
Tadalafil ODT Sildenafil ODT
Sildenafil ODT Tadalafil / Tramadol HCl Troches
Tadalafil / Tramadol HCl Troches Tadalafil / Apomorphine HCl Troches
Tadalafil / Apomorphine HCl Troches