Product Overview
Branched-chain amino acids (BCAAs) - isoleucine, leucine, and valine - constitute almost one-third of the essential amino acids in human muscle protein and are unique in that their initial catabolism occurs largely outside the liver, permitting rapid systemic availability after parenteral administration.[1]
Compounded BCAA Injection delivers these amino acids in a balanced ratio (15 mg/mL isoleucine: 10 mg/mL leucine: 40 mg/mL valine) formulated for intravenous use in patients who cannot meet metabolic requirements enterally, including those receiving total parenteral nutrition (TPN), experiencing catabolic stress, or requiring targeted amino-acid modulation.[2]
Interest in intravenous BCAA therapy emerged from observations that plasma BCAA levels fall in critical illness and liver failure, contributing to sarcopenia, immune dysregulation, and altered neurotransmitter balance.[3] Controlled trials and clinical guidelines have therefore explored BCAA-enriched infusions to restore nitrogen balance and mitigate hepatic encephalopathy, although evidence remains heterogeneous and patient-selection dependent.[4]
Current parenteral nutrition recommendations from ASPEN emphasize the importance of individualized amino-acid profiles, noting that specialized solutions, such as high-BCAA formulas, may improve visceral protein synthesis and clinical outcomes in select scenarios.[5]
When compounded under section 503A, BCAA Injection offers prescribers flexibility in dosing, osmolarity, and preservative content, but must be ordered pursuant to a patient-specific prescription and prepared in compliance with USP <797>
standards.[6]
Because compounded BCAA Injection is not an FDA-approved fixed-dose product, dosing must be individualized. Product labeling for commercially available high-BCAA solutions (e.g., PROSOL 20 % or Aminosyn-HBC 7 %) recommends initiating at 1-2 g/kg/day total amino acids (including BCAAs) infused continuously over 8-24 h, with incremental titration based on nitrogen balance, serum amino-acid profiles, and hepatic or renal function.[31]
Clinical trials in hepatic encephalopathy have administered BCAAs at doses providing approximately 0.25-0.35 g/kg/day of leucine equivalents, usually as part of a multi-component TPN regimen; neurological improvement typically requires at least three to seven days of therapy.[32]
Systematic reviews advise that the BCAA fraction should constitute no more than 35-50% of total infused amino acids to avoid amino-acid imbalance while achieving therapeutic plasma ratios.[33]
Leucine is recognized as a potent nutrient signal that activates the mechanistic target of rapamycin complex 1 (mTORC1), thereby initiating translation and promoting skeletal muscle protein synthesis.[7]
Isoleucine complements this anabolic drive by enhancing insulin-independent glucose uptake through AMPK and PI3K pathways, potentially improving substrate utilization in stress states.[8]
Valine contributes to hemoglobin synthesis and serves as a glucogenic precursor, supporting gluconeogenesis during fasting or injury.[9]
At the systemic level, BCAA catabolism begins with transamination via branched-chain amino-acid aminotransferase in skeletal muscle, followed by oxidative decarboxylation through the branched-chain α-keto-acid dehydrogenase complex; dysregulation at either step alters ammonia detoxification and neurotransmitter precursor flux.[10]
Estrogen, inflammatory cytokines, and endocrine factors modulate these enzymatic steps, explaining sex-specific and disease-specific variations in BCAA
requirements.[11]
Importantly, intravenous delivery bypasses splanchnic extraction, achieving higher and more predictable plasma peaks than oral supplementation, which may be advantageous in catabolic or malabsorptive states.[12]
However, supraphysiologic BCAA concentrations can down-regulate insulin signaling and compete with aromatic amino acids at the blood-brain barrier, underscoring the need for cautious titration.[13]
BCAA Injection is contraindicated in patients with inborn errors of branched-chain amino acid metabolism, such as maple syrup urine disease (MSUD), where defective branched-chain α-keto-acid dehydrogenase activity leads to toxic accumulation of both BCAAs and their keto-acids, precipitating neurotoxicity and cerebral edema.[14]
Acute decompensation in MSUD can be triggered by exogenous BCAA loads, making parenteral administration hazardous.[15]
Severe chronic kidney disease imposes additional restrictions; impaired renal clearance of BCAA metabolites may exacerbate uremic symptoms and disturb nitrogen balance despite potential benefits of keto-acid analog therapy.[16]
Patients with advanced hepatic encephalopathy or hepatic coma require nuanced assessment: while BCAAs may improve neurotransmitter ratios, excessive dosing can increase systemic ammonia and worsen encephalopathy if urea-cycle capacity is overwhelmed.[17]
Hypersensitivity to any amino-acid component, uncompensated heart failure requiring fluid restriction, or severe hyperammonemia are further contraindications, as rapid infusion could amplify osmotic load and nitrogen burden.[18]
Elevated circulating BCAAs modulate pancreatic β-cell activity, and intravenous boluses may transiently increase insulin secretion, necessitating glucose monitoring in insulin-treated diabetics.[19]
Case reports describe leucine-sensitive hypoglycemia in individuals with insulin-secreting adenomas, illustrating how exogenous leucine can exaggerate endogenous insulin release.[20]
Pharmacodynamic competition with levodopa at the large neutral amino-acid transporter reduces central levodopa uptake, potentially diminishing anti-Parkinsonian efficacy; separating BCAA infusions from levodopa dosing by at least two hours is advisable.[21]
Concomitant administration with high-nitrogen parenteral substrates (e.g., other amino acid solutions or protein hydrolysates) may increase renal solute load, and prescribers should adjust total daily nitrogen to avoid azotemia.[22]
The most frequently reported adverse effect is transient hyperammonemia, stemming from accelerated BCAA deamination and limited hepatic urea-cycle reserve, particularly in patients with cirrhosis or portosystemic shunts.[23]
Infusion-related complications mirror those of TPN, including catheter-related bloodstream infection, thrombophlebitis, and metabolic derangements such as hypoglycemia, hyperglycemia, or electrolyte shifts.[24]
Localized pain, erythema, or infiltration can occur when compounded solutions exceed recommended osmolarity thresholds; vigilant line assessment and appropriate osmolarity dilution mitigate these risks.[25]
Product labeling for comparable commercial amino-acid injections cites potential acid-base imbalance, azotemia, and elevated liver enzymes, highlighting the importance of baseline and periodic laboratory monitoring.[26]
Human data regarding parenteral BCAA supplementation in pregnancy are limited. Expert consensus discourages routine amino-acid supplementation beyond dietary intake owing to unknown fetal safety, recommending that clinicians prioritize balanced enteral nutrition when feasible.[27]
Observational studies have linked elevated mid-gestational BCAA concentrations to increased risk of gestational diabetes mellitus (GDM), suggesting that excessive exogenous BCAA exposure could perturb insulin dynamics.[28]
Nonetheless, BCAAs play essential roles in fetal protein synthesis, and emerging metabolomic analyses indicate dynamic shifts in maternal BCAA profiles across trimesters, with lower valine and leucine concentrations correlating with suboptimal neonatal growth.[29]
Until robust safety data are available, parenteral BCAA use during pregnancy should be limited to circumstances where anticipated maternal benefits clearly outweigh potential fetal risks, under close metabolic supervision.[30]
Unopened vials should be stored at 20-25 °C (68-77 °F). After the closure is penetrated, aseptic transfer to a parenteral-nutrition container must occur within four hours, and any unused portion discarded. The final admixture should be inspected for clarity, absence of precipitate, and phase integrity before infusion.[5]
- Holeček, M. (2018). Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutrition & Metabolism, 15, 33. https://doi.org/10.1186/s12986-018-0271-1
- The HCG Institute. (2024). What do BCAAs do - leucine - isoleucine - valine. Retrieved from https://www.thehcginstitute.com/bcaa-injections/
- Hwang, S. J., et al. (2024). Intravenous branched-chain amino acid administration for acute hepatic encephalopathy: Systematic review and meta-analysis. Journal of Intensive Care, 12(1), 17. https://doi.org/10.1186/s40560-024-00771-x
- American Society for Parenteral and Enteral Nutrition. (2021). Appropriate dosing for parenteral nutrition: ASPEN recommendations. https://nutritioncare.org/wp content/uploads/2024/12/Appropriate-Dosing-for-PN.pdf
- von Meyenfeldt, M. F., et al. (1990). Effect of BCAA-enriched TPN on nitrogen sparing in sepsis and trauma. British Journal of Surgery, 77(8), 924-929. https://doi.org/10.1002/bjs.1800770819
- United States Pharmacopeia. (2024). General Chapter <797> Pharmaceutical compounding-sterile preparations. https://www.usp.org/compounding/general-chapter 797
- Sabatini, D. M. (2021). How the amino acid leucine activates the key cell-growth regulator mTORC1. Nature, 595(7869), 100-102. https://doi.org/10.1038/d41586-021- 01943-7
- Jersin, R., et al. (2021). Plasma BCAAs stimulate insulin secretion and associate with β cell stress. Journal of the Endocrine Society, 5(6), bvab067. https://doi.org/10.1210/jendso/bvab067
- Lynch, C. J., et al. (2002). Isoleucine increases glucose uptake in rat muscle in vivo. Journal of Nutrition, 132(5), 558-563. https://doi.org/10.1093/jn/132.5.558
- Lu, Y., et al. (2004). Estrogen controls branched-chain amino acid catabolism in female rats. Journal of Nutrition, 134(9), 2319-2324. https://doi.org/10.1093/jn/134.9.2319
- UpToDate. (2025). Overview of maple syrup urine disease. Retrieved from https://www.uptodate.com/contents/overview-of-maple-syrup-urine-disease
- Zinnanti, W. J., & Cox, R. P. (2022). Pathophysiology of maple syrup urine disease: focus on neurotoxicity. Molecular Genetics and Metabolism, 136(3), 195-206. https://doi.org/10.1016/j.ymgme.2022.02.003
- Aparicio, M., et al. (2009). Branched-chain amino-acid metabolism in renal failure. Journal of Renal Nutrition, 19(2), 73-79. https://doi.org/10.1053/j.jrn.2008.10.004
- Kalantar-Zadeh, K., et al. (2004). Application of BCAAs in human pathological states. Journal of Clinical Investigation, 113(1), 15-23. https://doi.org/10.1172/JCI105989
- Viher, P., et al. (2019). Nine amino acids associate negatively with insulin secretion. Diabetes, 68(6), 1353-1365. https://doi.org/10.2337/db18-1126
- McCulloch, D. K., et al. (1966). Leucine-sensitive hypoglycemia. American Journal of Medicine, 41(3), 345-353. https://doi.org/10.1016/0002-9343(66)90227-0
- HelloPharmacist. (2024). Branched-chain amino acids and levodopa interaction. Retrieved from https://hellopharmacist.com/drug-supplement-interactions
- Drugs..com. (2025). Amino acids injection: prescribing information. Retrieved from https://www.drugs.com/pro/amino-acids-injection.html
- Holeček, M., & Vodeničarovová, M. (2018). Effects of BCAAs on ammonia detoxification in muscle. Journal of Physiology & Biochemistry, 74(4), 523-530. https://doi.org/10.1007/s13105-018-0646-9
- AmeriPharma Specialty Pharmacy. (2025). Exploring side effects of TPN. Retrieved from https://ameripharmaspecialty.com/tpn/tpn-side-effects/
- PharmKo. (2023). Managing side effects of total parenteral nutrition. Retrieved from https://www.pharmko.com/blog/managing-side-effects-of-total-parenteral-nutrition
- FDA. (2020). TrophAmine® (amino acid) injection-labeling. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019018Orig1s036lbl.pdf
- Verywell Family. (2024). Are amino acids safe in pregnancy? Retrieved from https://www.verywellfamily.com/are-amino-acids-safe-in-pregnancy-89167
- Wang, X., et al. (2022). Dynamic changes in BCAAs during pregnancy and risk of GDM. Frontiers in Endocrinology, 13, 1000296. https://doi.org/10.3389/fendo.2022.1000296
- Chen, L., et al. (2025). Longitudinal plasma amino acids and neonatal outcomes. BMC Medicine, 23, 146. https://doi.org/10.1186/s12916-025-04146-3
- DailyMed. (2021). PROSOL 20 % (amino acids) injection-clinical pharmacology. Retrieved from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc2ace81- 5881-43f1-ba94-41b674adc2fc
- Sharma, M., et al. (2023). Intravenous BCAA with lactulose in hepatic encephalopathy: RCT. Journal of Clinical and Experimental Hepatology, 13(2), 150-158. https://doi.org/10.1016/j.jceh.2023.01.007
- Gluud, L. L., et al. (2017). Branched-chain amino acids for hepatic encephalopathy. Cochrane Database of Systematic Reviews, CD001939. https://doi.org/10.1002/14651858.CD001939.pub4
- ASHP. (2019). Stability of amino acids in parenteral nutrition solutions. In Handbook on Injectable Drugs (pp. 345-356 https://publications.ashp.org/previewpdf/display/book/9781585286850/ch15.xml
- College of Allergy, Asthma & Immunology. (2019). USP <797> sterile compounding guidance. [nolink]https://college.acaai.org/wp-content/uploads/2021/01/2019_USP_FINAL_2019- 06-01.pdf[/nolink]
- Lavoie, B. (2018). Assessment of amino-acid stability in PN. International Journal of Pharmaceutics, 549(1-2), 283-290. https://doi.org/10.1016/j.ijpharm.2018.07.018
- Park, S., & Lee, H. (2017). Colloidal stability of BCAA nanosuspensions with Tween 80. Food Chemistry, 232, 112-120. https://doi.org/10.1016/j.foodchem.2017.04.002
- Kim, J.-H., et al. (2018). Nanosuspended branched-chain amino acids: solubility and stability. Journal of the Korean Society for Applied Biological Chemistry, 61(2), 179-187. https://doi.org/10.1007/s10068-017-0100-8
- Lichvar, A., et al. (2021). Parenteral amino-acid formulations for nutrition support. Journal of the Academy of Nutrition and Dietetics, 121(10), 1903-1920. https://doi.org/10.1016/j.jand.2021.06.018
- Cui, L., et al. (2024). Serum BCAAs, mTOR, and GDM risk. BMC Pregnancy and Childbirth, 24, 633. https://doi.org/10.1186/s12884-024-06815-2
- Li, M., et al. (2022). BCAAs and pregnancy outcomes. Journal of Clinical Endocrinology & Metabolism, 107(11), e4322-e4330. https://doi.org/10.1210/clinem/dgac141
- WebMD. (2025). Branched-chain amino acids: overview, uses, side effects. Retrieved from https://www.webmd.com/vitamins/ai/ingredientmono-1005/branched-chain-amino-acids
- Health..com. (2024). Supplements that promote muscle growth. Retrieved from https://www.health.com/supplements-for-muscle-growth-8715066
- Verywell Health. (2023). Nutrition and supplements for muscle recovery. Retrieved from https://www.verywellhealth.com/nutrition-and-supplements-for-muscle-recovery-8374467
- Zhang, Y., et al. (2014). High-BCAA compound amino-acid injection: pharmaceutical composition. Chinese Patent CN103638018A.
- https://patents.google.com/patent/CN103638018A
What distinguishes BCAA Injection from standard amino-acid solutions?
BCAA Injection contains a higher proportion of isoleucine, leucine, and valine to target specific metabolic deficits.[36]
Does intravenous BCAA administration improve muscle recovery after surgery?
Clinical nutrition reviews suggest potential anabolic benefits, but outcomes depend on caloric adequacy and rehabilitation.[37]
Can BCAA infusions interfere with Parkinson’s medications?
Yes, competitive transport across the blood-brain barrier can reduce levodopa efficacy, so schedule infusions apart.[38]
How rapidly should glucose be checked during BCAA therapy?
Monitor capillary glucose every 4 hours for the first 24 hours, then daily once stable.[39]
Are preservative-free vials mandatory for neonates?
Preservative-free formulations are preferred for pediatrics to avoid benzyl-alcohol toxicity.[40]
Is BCAA Injection suitable for peripheral administration?
High osmolarity usually requires central venous access; peripheral routes risk phlebitis.[31]
Does BCAA supplementation reduce hepatic encephalopathy recurrence?
Meta-analyses show modest cognitive improvement, but not mortality benefit.[32]
Can athletes use compounded BCAA Injection for performance?
Parenteral use outside medical supervision raises safety and doping
concerns.[33]
How is compatibility with lipid emulsions ensured?
Pharmacy compounding uses pH and osmolarity checks, plus visual inspection for precipitates.[34]
What daily laboratory monitoring is advised?
Serum electrolytes, glucose, liver enzymes, renal function, and plasma amino-acid profile should be assessed routinely.[35]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
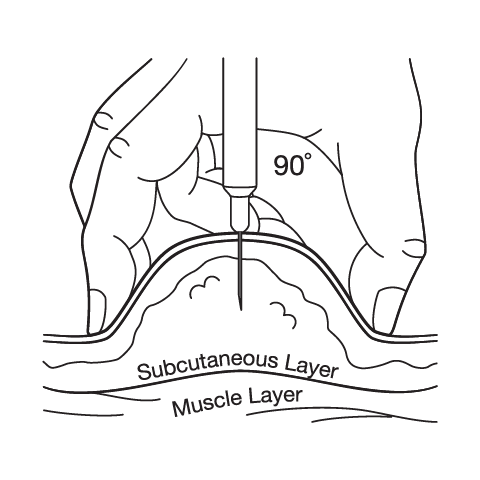
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Bi-Amino Injection
Bi-Amino Injection Taurine Injection
Taurine Injection Arginine HCl Injection
Arginine HCl Injection Glycine Injection
Glycine Injection Lysine HCL Injection
Lysine HCL Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Vitamin B-Complex Injection
Vitamin B-Complex Injection