Product Overview
Sermorelin acetate is a synthetic analog of growth hormone-releasing hormone (GHRH) consisting of the first 29 amino acids of natural human GHRH. It stimulates the pituitary gland to release endogenous growth hormone (GH).[1]
By increasing GH secretion, sermorelin can raise insulin-like growth factor 1 (IGF-1) levels in the body.[2] This therapy is used primarily for diagnosed growth hormone deficiency, for example, in children who fail to grow due to inadequate GH production.[1] In adults with confirmed GH deficiency, sermorelin may also be considered as a treatment option, although its use in healthy older adults for “anti-aging” purposes remains unproven and is not an FDA-approved indication.[3]
Sermorelin was originally approved in the 1990s for GH deficiency but was later discontinued as a commercial drug for business reasons (not due to safety or efficacy concerns).[4] It is now available as a compounded medication prepared by specialty pharmacies.
Sermorelin is administered via subcutaneous injection, often once daily at night, and must be prescribed by a qualified healthcare provider. Its mechanism of action, stimulating the patient’s own GH release, offers a more physiologic alternative to direct recombinant GH injections, potentially with a different side effect profile.
All use of sermorelin should be under medical supervision with appropriate monitoring of efficacy and safety parameters.
General Dosing Information: The dosage of sermorelin must be individualized based on the patient’s age, weight (particularly in pediatric patients), and the condition being treated. Therapy should be supervised by a physician experienced in managing growth hormone disorders. The following are typical dosing guidelines:
- Therapeutic Dose (Adults): A common adult dosing regimen for growth hormone deficiency is 0.2 to 0.3 mg of sermorelin acetate injected subcutaneously once daily at bedtime.[13] Dosing at night is thought to mimic the natural physiologic rhythm, as endogenous GH release is highest during sleep. The injection is given into the fatty subcutaneous tissue (often in the abdomen, thigh, or upper arm). The healthcare provider will determine the appropriate starting dose; some clinicians may start at the lower end (0.2 mg) and adjust based on IGF-1 levels and clinical response.
- Therapeutic Dose (Pediatrics): In children with idiopathic GH deficiency, sermorelin has historically been dosed according to body weight. A typical pediatric dose is approximately 0.03 mg per kilogram of body weight (30 µg/kg) once daily at bedtime.[5] For example, a 20-kg child might receive around 0.6 mg nightly. This weight-based dosing is designed to stimulate adequate growth in growing children. Clinicians monitor growth velocity and IGF-1 levels and may adjust the dose over time. It’s important to note that in pediatric studies, sermorelin’s efficacy was variable; some children showed sustained increases in growth velocity over 12-36 months of therapy, while others responded less robustly (somatropin GH is generally more potent).[5] If a child does not respond to sermorelin, alternative treatments may be needed.
- Diagnostic Dose (Provocative Testing): In endocrinology practice, sermorelin (GHRH 1-29) can be used as a diagnostic agent to test pituitary GH reserve. For this purpose, an intravenous dose of 1.0 µg/kg is administered under medical supervision, and GH levels are measured in the blood at intervals thereafter.[1] A normal pituitary will release a surge of GH in response. This diagnostic use is less common today, but may be seen in specialized testing. (This dosing is mentioned for completeness; diagnostic testing should only be done by healthcare professionals in a clinical setting.)
Administration Details: Sermorelin is supplied as a lyophilized powder in sterile vials that require reconstitution with the provided diluent (usually bacteriostatic water for injection). Patients or caregivers should be instructed thoroughly on how to prepare the injection.
The contents of the vial are mixed gently with the diluent (avoiding shaking, which can denature the peptide) until fully dissolved.[13] The appropriate dose is then drawn up into an insulin-type syringe.
Injections are given subcutaneously (just under the skin), and injection sites should be rotated daily to prevent lipoatrophy or irritation. Common injection areas include the lower abdomen (at least 2 inches away from the navel), front of the thigh, or outer upper arm.
The evening timing (before bedtime) is recommended to leverage the body’s natural night-time GH peak and to reduce daytime inconvenience.
If a dose is missed, the patient should follow their physician’s guidance; generally, if it’s close to the next dose, skip the missed dose (do not double up doses).
All used needles and syringes must be disposed of in a proper sharps container.
Treatment Duration and Monitoring: The length of sermorelin therapy depends on the individual’s response and treatment goals. In children, therapy may continue for several years until an acceptable height is achieved or until growth plates close. In adults, treatment length varies based on symptom relief and IGF-1 targets, and some may use it indefinitely if benefits persist (with periodic re-evaluation).
During therapy, doctors will regularly monitor IGF-1 levels in the blood to gauge the biological response to sermorelin. Dose adjustments may be made to keep IGF-1 within an appropriate target range for age. Other parameters like growth velocity (in children), body composition, bone density, or general well-being can help assess effectiveness.
Safety monitoring includes periodic checks of blood glucose, thyroid function, and adrenal function, as well as watching for any adverse effects. If the desired results are not seen after 3-6 months, or if the patient cannot tolerate the medication, discontinuation or alternative treatments may be considered.
Note: All dosing should follow the prescriber’s orders. The above doses are average recommendations; physicians may tailor the dose outside these ranges based on the clinical scenario. Never change the dose or frequency without consulting the prescribing healthcare provider.
Sermorelin mimics the action of endogenous GHRH on the pituitary GHRH receptors, thereby promoting the synthesis and pulsatile release of growth hormone from the anterior pituitary gland.[5] In essence, it “releases the brake” on the pituitary, causing a surge in the patient’s own GH output.
Following a sermorelin dose, the increase in circulating GH leads to a corresponding rise in IGF-1 production primarily from the liver, which mediates many of the growth-promoting and metabolic effects of GH.[6]
Sermorelin’s activity closely resembles that of natural GHRH, so it induces GH release in a physiologic (pulsatile) manner.[5] Notably, normal feedback mechanisms remain intact: elevated IGF-1 and GH can signal the hypothalamus and pituitary to modulate further hormone release, which may reduce the risk of extreme hormone levels.
By engaging this natural endocrine loop, sermorelin therapy may support improvements in lean body mass, bone density, and other parameters in GH-deficient individuals; although responses vary and are generally less pronounced than with direct GH administration.
Importantly, sermorelin is effective only if the pituitary gland is capable of producing GH; it will not elicit a response in patients with absent or nonfunctional somatotroph cells.
Sermorelin should not be used in patients with certain conditions where the risks outweigh potential benefits. Key contraindications and precautions include:
- Hypersensitivity: Any history of allergy to sermorelin or to any of the formulation’s excipients is a contraindication to therapy.[7] Signs of an allergic reaction (such as rash, hives, or anaphylaxis) preclude further use.
- Active Malignancy: Because growth hormone can promote cell proliferation, sermorelin is contraindicated in patients with active cancers or tumors. GH stimulation may theoretically encourage tumor growth, so this therapy is avoided in anyone with an active malignancy or a history of cancer that is not in complete remission.[8] (Patients in long-term remission should only be considered for therapy on a case-by-case basis in consultation with an oncologist.)
- Intracranial Lesions: Sermorelin is not recommended for patients whose GH deficiency is caused by an intracranial tumor or lesion. Clinical trials of sermorelin did not include patients with pituitary tumors or other brain lesions causing hormone deficiencies, so safety and efficacy in this group are not established.[9] Alternative treatments should be considered in such cases.
- Uncontrolled Hypothyroidism: An underactive thyroid must be managed before starting sermorelin. Untreated hypothyroidism blunts the physiological response to sermorelin, as normal thyroid hormone levels are needed for optimal GH production.[10] Patients should have thyroid function assessed and corrected prior to and during sermorelin therapy to ensure effectiveness.
- Pregnancy: Use of sermorelin in pregnancy is not advised (see Pregnancy section below). Women who are pregnant or planning to become pregnant should avoid this medication unless clearly needed, due to unknown fetal risks.[11]
- Lactation: Caution is urged in breastfeeding mothers (see Pregnancy section). It is not known if sermorelin is excreted in human milk.[12]
All patients should be evaluated by a healthcare professional to ensure none of the above contraindications or precautions apply.
Sermorelin therapy is indicated only for individuals with demonstrated need (such as GH deficiency); it is not intended for use in patients with normal GH levels, and it is legally prohibited to distribute GH or its secretagogues for anti-aging or athletic enhancement purposes.
Providers will weigh the potential risks (e.g. tumor growth, metabolic effects) against benefits before initiating therapy in each patient.
Before starting sermorelin, patients should inform their healthcare provider of all medications and supplements they are taking, as some can interfere with sermorelin’s efficacy or safety. Notable drug interactions and related considerations include:
Glucocorticoids: Concurrent high-dose glucocorticoid (corticosteroid) therapy may inhibit the GH response to sermorelin.[6] Steroids such as prednisone can suppress pituitary growth hormone release, potentially reducing sermorelin’s effectiveness. Patients on chronic corticosteroids may require dose adjustments or careful monitoring of IGF-1 levels to ensure sermorelin is having the desired effect.
Somatostatin Analogs: Medications that mimic somatostatin (the hormone that opposes GH release) can counteract sermorelin. For example, octreotide (used in acromegaly or certain tumors) or other drugs that increase somatostatin activity may blunt the GH rise from sermorelin. These combinations should generally be avoided or approached with caution, as sermorelin may be ineffective if somatostatin analogs are on board.
Thyroid Medications: The thyroid status of the patient significantly influences sermorelin’s activity. As noted, untreated hypothyroidism will interfere with sermorelin’s effects.[10] Conversely, starting or stopping thyroid hormone replacement could alter the growth hormone response. While levothyroxine itself is not a direct antagonist to sermorelin, thyroid levels need to be stable. In addition, anti-thyroid drugs (e.g. propylthiouracil) that induce a hypothyroid state may diminish sermorelin’s efficacy.[13] Close coordination of thyroid treatment with sermorelin therapy is recommended.
Insulin and Blood Sugar: GH can antagonize insulin’s actions, so diabetics on insulin or oral hypoglycemics should be closely monitored when initiating sermorelin. Although not a direct “drug-drug interaction,” increased GH/IGF-1 levels may raise blood glucose. Adjustments to diabetes medications might be needed to maintain glycemic control. Patients should report significant changes in blood sugar to their provider.
Other Medications: Certain central nervous system medications used in GH stimulation tests, for example, dopamine agonists (like L-Dopa) or arginine, can affect GH release. While these are typically only relevant in diagnostic settings, any drug affecting pituitary function or hormone levels could theoretically impact sermorelin therapy.
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as high-dose aspirin or indomethacin have been reported to alter GH release in some contexts, though this is more pertinent to GH stimulation testing than chronic therapy.[13]
In summary, any medication that significantly alters pituitary-hypothalamic function or hormonal balance should be brought to the attention of the prescribing practitioner.
Lab test interactions: Note that sermorelin can cause transient changes in certain lab values; for instance, it may increase serum levels of markers like inorganic phosphorus or alkaline phosphatase.[9] Healthcare providers and laboratory personnel should be aware the patient is on sermorelin, so that lab results are interpreted appropriately. As always, patients are advised not to start or stop any other prescription drug, over-the-counter product, or supplement without consulting their healthcare provider during sermorelin therapy.
Common Side Effects: The most frequent adverse effects of sermorelin are related to its injection route. Approximately one in six patients (up to ~16 %) experience localized reactions at the injection site.[4] These reactions may include pain, swelling, redness, or mild bruising at the site of the subcutaneous injection. Such local side effects are usually transient and self-limited. Rotating injection sites with each dose can help minimize irritation. Patients should be taught proper injection technique to reduce the risk of injection-site injury or infection.
Systemic Side Effects: Sermorelin is generally well tolerated, and because it triggers endogenous hormone release, systemic side effects tend to be mild for most patients. Possible side effects include: headache, flushing (warmth or redness of the face), dizziness or light-headedness, nausea, or transient fatigue/somnolence.[4] Some individuals report a temporary feeling of restlessness or hyperactivity, while others may feel sleepy after the injection; these responses vary. A temporary “strange” or metallic taste in the mouth has also been noted in some cases immediately after injection, but this is not harmful.[13] Most of these reactions, if they occur, subside as the body adjusts to therapy.
Rare or Serious Adverse Reactions: Serious reactions to sermorelin are uncommon. Unlike recombinant GH therapy, sermorelin has not been strongly associated with severe fluid retention, insulin resistance, or joint pain in the short term, though long-term data are limited. Rarely, some patients may experience difficulty swallowing or the sensation of a lump in the throat (dysphagia) after injections; if this occurs, medical advice should be sought, as it could signify an allergic or unusual response.[4] Allergic reactions to sermorelin are possible (as with any peptide). While no widespread anaphylactic reactions were reported in clinical trials, there has been an isolated case of a severe local hypersensitivity reaction characterized by intense redness, swelling, and urticaria (hives) at injection sites in a patient who developed anti-sermorelin antibodies.[4] Patients should stop the medication and seek prompt medical care if they experience generalized rash, hives, chest tightness, or difficulty breathing.
Immunogenicity: A notable proportion of patients (approximately 70 % or more) may develop low-titer antibodies to sermorelin with chronic use.[4] These are antibodies directed against the GHRH analog. However, current evidence suggests that the development of such antibodies does not significantly reduce the drug’s effectiveness or cause any distinct adverse reaction profile.[4] In other words, most patients with detectable anti-sermorelin antibodies continue to respond to therapy, and the antibodies may disappear over time even if treatment continues. Nonetheless, healthcare providers may monitor for any signs of reduced efficacy over the long term.
Metabolic Considerations: Because sermorelin increases GH and IGF-1 levels, it may have subtle effects on metabolism that warrant attention. In some patients, insulin sensitivity may be reduced, as GH tends to raise blood glucose by antagonizing insulin. While frank hyperglycemia is uncommon, those with underlying insulin resistance or diabetes should be monitored for any change in glucose control. Likewise, sermorelin therapy can unmask hypothyroidism in susceptible individuals; clinical studies in children noted a small percentage of patients developed hypothyroidism during GH/GHRH therapy and required thyroid hormone replacement.[10] For this reason, periodic thyroid function tests are recommended, and any emerging thyroid deficiency should be treated. Unlike high-dose GH administration, sermorelin is not known to cause significant fluid retention, carpal tunnel syndrome, or organomegaly in the doses used for deficiency treatment; it aims to restore normal physiologic levels rather than create supraphysiologic levels.
Patients are encouraged to report any persistent or concerning symptoms to their healthcare provider, even if not listed here. Most side effects of sermorelin are mild, but ongoing monitoring (including periodic blood tests for IGF-1 and other parameters) is part of therapy to ensure safety. If side effects are troublesome, dose adjustments or discontinuation may be considered. Overall, when used in appropriate patients under medical supervision, sermorelin is typically well tolerated, and serious adverse effects are rare.[5]
Pregnancy: Sermorelin is categorized as Pregnancy Category C (under the older FDA classification system).[11] This indicates that animal reproduction studies have shown an adverse effect on the fetus, and there are no adequate, well-controlled studies in pregnant women. In animal studies, very high doses of sermorelin (several times the human dose) produced minor variations in fetal development in rats and rabbits.[11] It is not known whether sermorelin can cause fetal harm when administered to a pregnant woman or if it can affect reproductive capacity. Use in pregnancy is not recommended unless the potential benefit justifies the potential risk to the fetus.[11]
In practice, sermorelin is almost never used during pregnancy, since treating chronic growth hormone deficiency can typically be deferred and other therapies (if needed) would be chosen if GH support was truly required. Women of childbearing potential should inform their physician if they become pregnant or intend to become pregnant while on sermorelin. If pregnancy occurs, the healthcare provider will likely discontinue sermorelin due to the lack of safety data.
Breastfeeding: It is not known whether sermorelin acetate is excreted in human breast milk.[12] Because many drugs and small peptides can be present in breast milk, caution is advised. There is also insufficient information on the effects of sermorelin on a nursing infant. Given the essential role of GH/IGF-1 in growth and metabolism, any exposure of a newborn to sermorelin via milk is of uncertain significance.
Therefore, breastfeeding is generally not advised while receiving sermorelin, or the drug should be used only if clearly needed and if the mother’s healthcare provider determines that the benefits outweigh the potential risks to the infant.[12] Nursing mothers on sermorelin should closely collaborate with their healthcare providers; an alternative feeding plan for the infant (or alternative therapy for the mother) may be recommended to ensure safety.
In summary, women who are pregnant, breastfeeding, or planning to become pregnant should discuss thoroughly with their doctors before considering sermorelin. In almost all cases, sermorelin therapy will be avoided during pregnancy and lactation to err on the side of caution, unless there are special circumstances and a consensus that treatment is necessary. All decisions should be individualized and made in conjunction with an obstetrician or maternal-fetal medicine specialist as appropriate.
Proper storage of sermorelin is vital to maintain its stability and potency. This compounded peptide must be handled and stored according to pharmacy instructions and general guidelines for biopharmaceuticals:
Dry Powder Storage: Unmixed (dry) sermorelin vials are to be stored at room temperature 20-25°C (68-77°F). Keep the medication in its original vial and box until use, to protect it from light. Do not freeze the vials, as freezing can damage the peptide structure. Do not expose sermorelin to high heat or direct sunlight.
After Reconstitution: Once you have mixed (reconstituted) sermorelin with diluent, it must be kept refrigerated at 2-8°C and should be used within the time frame specified by the pharmacy. Under refrigerated conditions, the reconstituted solution is typically stable for a limited period (often 1-2 weeks for many compounded peptides, though some preparations may remain stable up to 28 days or longer; follow your compounding pharmacy’s beyond-use date). Because no preservatives other than bacteriostatic water are present, sterility and potency beyond the recommended usage period cannot be guaranteed. It is usually advised to mark the date of mixing on the vial and discard any unused portion after the indicated expiration or beyond-use date.
Handling Precautions: Before mixing and drawing up doses, wash hands and swab the vial stopper with alcohol to maintain sterility. Use only the diluent provided (or recommended) for reconstitution. Do not shake the vial vigorously; gentle swirling is sufficient to dissolve the powder. Inspect the solution: if it is discolored or contains particulate matter that does not dissolve, do not use it. Prepare injections on a clean flat surface.
Disposal: Keep all medication and supplies out of reach of children and pets. Dispose of used needles and syringes in a FDA-cleared sharps disposal container; never in the regular trash. For any unused or expired sermorelin vials, follow pharmacy instructions for disposal. Do not flush medications down the toilet or pour them into drains. If you need to dispose of medication and do not have a take-back program or instructions, mix the residual drug with an unpalatable substance (like coffee grounds or cat litter), seal it in a bag, and discard in the trash, per FDA guidelines, to prevent accidental exposure.
Always refer to the specific storage guidelines provided with your compounded prescription, as formulation variations can exist. Some compounded sermorelin products may specify storage at room temperature prior to mixing; but in general, keeping the product refrigerated is safest to ensure maximum shelf life and efficacy.[14]
If you have any doubts about whether the medication has been stored properly or if it was left unrefrigerated for an extended time, contact the pharmacy or your healthcare provider for advice. Proper storage and handling will help maintain sermorelin’s effectiveness throughout your treatment course.
- Mayo Clinic. (2025). Sermorelin (injection route) - Description. In Drugs and Supplements. Mayo Foundation for Medical Education and Research. Retrieved from https://www.mayoclinic.org/drugs-supplements/sermorelin-injection-route/description/drg-20065923
- ScienceDirect. (n.d.). Sermorelin - an overview | ScienceDirect Topics. ScienceDirect (Elsevier). Retrieved May 2, 2025, from https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/sermorelin (Content notes FDA approval 1997 and withdrawal 2008 for commercial reasons.)
- Askinazi, O. (2023, August 21). Sermorelin Therapy: Benefits, Uses, Side Effects, and More. Healthline. Retrieved from https://www.healthline.com/health/sermorelin
- Prakash, A., & Goa, K. L. (1999). Sermorelin: A review of its use in the diagnosis and treatment of children with idiopathic growth hormone deficiency. BioDrugs, 12(2), 139-157. https://doi.org/10.2165/00063030-199912020-00007
- RxList. (2001). Sermorelin Acetate - Clinical Pharmacology & Adverse Reactions (FDA Prescribing Information, rev. Oct 2001). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- RxList. (2001). Sermorelin Acetate - Drug Interactions and Usage (FDA Prescribing Information, rev. Oct 2001). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- RxList. (2001). Sermorelin Acetate - Contraindications & Precautions (FDA Prescribing Information, rev. Oct 2001). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- Eledrisi, M. S. (2022, June 17). Growth Hormone Deficiency in Adults - Treatment & Management. Medscape eMedicine. Retrieved from https://emedicine.medscape.com/article/120767-treatment (GH therapy contraindicated in active malignancy)
- RxList. (2001). Sermorelin Acetate - Warnings and Laboratory Tests (FDA Prescribing Information). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- RxList. (2001). Sermorelin Acetate - Precautions (Hypothyroidism). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- RxList. (2001). Sermorelin Acetate - Use in Specific Populations (Pregnancy). RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- RxList. (2001). Sermorelin Acetate - Nursing Mothers. RxList (WebMD). Retrieved from https://www.rxlist.com/sermorelin-acetate-drug.htm
- Drugs.com. (2018). Sermorelin acetate - Consumer Information. Drugs.com. Retrieved from https://www.drugs.com/cdi/sermorelin-acetate.html (Interactions and administration tips)
- Drugs.com. (2020). Sermorelin Storage and Stability. Drugs.com. Retrieved from https://www.drugs.com (Refrigeration between 2-8°C is recommended for sermorelin vials)
What is sermorelin and why is it prescribed?
Sermorelin is a synthetic peptide that stimulates your body’s release of growth hormone. It is prescribed to treat growth hormone deficiency or related conditions. By prompting the pituitary gland to produce more GH, sermorelin can help increase IGF-1 levels, which in turn may improve growth in children or address symptoms of adult GH deficiency.
It is used under medical supervision when a patient’s natural GH levels are abnormally low. Sermorelin is not a steroid or actual growth hormone; rather, it “kick-starts” your own hormone production. Doctors prescribe it for specific medical indications, for example, children with poor growth due to low GH, or adults with confirmed GH deficiency causing symptoms.
It is not intended for general use in healthy individuals. A valid prescription is required, and therapy typically involves daily injections along with regular monitoring by the healthcare provider.
How does sermorelin differ from human growth hormone (hGH) therapy?
Both sermorelin and hGH (somatropin) aim to increase the levels of growth hormone effects in the body, but they work in different ways. Sermorelin is a growth hormone-releasing hormone analog; it tells your pituitary to release your own GH. This approach can restore a more natural, pulsatile hormone rhythm. hGH therapy, on the other hand, involves injecting recombinant human growth hormone directly.
Sermorelin relies on a functional pituitary gland; if your pituitary cannot produce GH, sermorelin won’t be effective (in such cases, hGH injections would be needed). In terms of safety, sermorelin’s effects are considered more physiologic; your body can still regulate GH output to some degree (for example, if IGF-1 gets too high, feedback mechanisms can reduce further GH release).
With direct hGH injections, especially at high doses, there’s a risk of excessively elevated GH/IGF-1 levels, which may lead to side effects like joint pain, edema, insulin resistance, or carpal tunnel syndrome. Sermorelin’s side effect profile is mainly limited to injection-site reactions and transient symptoms, whereas hGH can have broader systemic effects if not dosed carefully.
Importantly, sermorelin is only useful for individuals who have an ability to make GH but need a boost; hGH therapy replaces the hormone outright. Doctors will choose one or the other based on the patient’s specific condition.
In pediatric growth hormone deficiency, hGH injections are more commonly used and generally more potent, but sermorelin was an FDA-approved alternative in the past. Some adults who cannot legally obtain hGH (which is tightly controlled) might be prescribed sermorelin.
In summary: Sermorelin stimulates natural GH release (acting upstream), while hGH therapy provides external GH (acting downstream). Each has its role, and the doctor will recommend the best option for the patient’s situation.
Who should avoid taking sermorelin?
Individuals with certain conditions should not use sermorelin. If you are pregnant or breastfeeding, you should avoid sermorelin because its safety for unborn babies or nursing infants is not established. Patients with active cancer (any malignancy) must not use sermorelin, since increasing growth factors could potentially stimulate tumor growth.
Anyone with a known allergy to sermorelin or any ingredient in its formulation should not take it; if you’ve had a hypersensitivity reaction to similar medications, inform your doctor. Additionally, if you have a pituitary tumor or other brain lesion causing your hormone problems, sermorelin may not be appropriate; in such cases, direct treatment of the tumor or use of different therapy is needed instead.
People with untreated hypothyroidism should delay sermorelin until their thyroid condition is managed, because low thyroid hormone levels can make sermorelin ineffective (thyroid hormone is important for growth hormone action). Finally, sermorelin is not meant for those who do not actually have GH deficiency. If you have normal GH levels for your age, the risks of taking sermorelin outweigh any unproven benefits; it’s not a general wellness or anti-aging drug.
Always review your full medical history with your healthcare provider. Conditions like uncontrolled diabetes or intracranial hypertension, for example, might also warrant caution or closer monitoring if sermorelin is used. Your doctor will screen for contraindications and decide if sermorelin is safe for you. When in doubt, it’s crucial to follow medical advice and not to use sermorelin in scenarios for which it is not intended.
How is sermorelin given, and can I self-administer it at home?
Sermorelin is given as a subcutaneous injection (an injection under the skin into fatty tissue). It comes as a powder that must be mixed with a diluent (usually bacteriostatic water) to form a solution. Many patients do learn to self-administer sermorelin at home after proper instruction. Typically, you would inject sermorelin once a day at bedtime.
The process involves using an insulin-style syringe to inject a small volume (often 0.5 mL or less) into a spot like your lower abdomen or thigh. Your healthcare provider or a nurse will teach you the correct mixing technique, how to draw up the dose, and how to inject safely. It’s important to rotate injection sites to prevent skin irritation, for example, alternate between left and right abdomen or different areas of the thigh each night.
Storage is critical: you’ll keep the vial refrigerated and protected from light (see Storage guidelines above). Before each injection, you clean the vial top with alcohol, use a new sterile needle, and inject the water into the powder vial to reconstitute it. Gently swirl until dissolved (never shake hard). Then withdraw your prescribed dose.
Pinch a fold of skin, insert the needle at about a 45-degree angle, and inject the solution. Dispose of the needle properly in a sharps container. If you are uncomfortable with injections, discuss this with your provider; they can offer additional training or see if an alternative therapy exists.
Many patients find the tiny insulin syringe needle to be very manageable, as it’s quite small. Over time, self-injection becomes routine for most people. Always double-check the dose and instructions given by your doctor. Do not attempt to adjust the dose on your own.
If you ever miss a dose or have an administration error (for instance, you spill the medication), contact your healthcare provider for guidance on what to do next. With proper education and precautions, administering sermorelin at home is feasible and safe for the majority of patients who require it.
What are the common side effects, and what should I do if I experience them?
The most common side effects of sermorelin are relatively mild. You might notice redness, itching, pain, or swelling at the injection site. This is usually not serious; applying a cool compress to the area can help, and these local reactions typically resolve on their own. Rotating injection sites each time helps minimize this issue.
Other side effects some people report include headache, a brief feeling of flushing or warmth, or nausea shortly after the injection. These symptoms, if they occur, tend to be transient. You might also feel a bit light-headed or tired for a short while. In most cases, these effects don’t require medical intervention; you can mention them to your doctor at your next visit.
However, if any side effect is intense or worrying, you should contact your healthcare provider right away. For example, if you develop a rash, hives, or wheezing (signs of an allergic reaction), or if you feel chest tightness or have difficulty swallowing, seek medical attention immediately; those could be signs of a rare allergic response. Another uncommon side effect is dysphagia (trouble swallowing) or a lump-in-throat sensation; if you experience that, let your doctor know promptly.
Over the longer term, because sermorelin raises growth hormone levels, your doctor will watch for signs of fluid retention, joint pain, or changes in blood sugar; though these are more typical with high-dose GH therapy than with sermorelin. It’s important to keep all follow-up appointments and blood test labs as instructed so any subtle side effects can be caught early.
In summary: most people tolerate sermorelin well. Expect possibly some injection-site soreness and occasionally mild headache or flushing. These can often be managed with simple measures (e.g., analgesic for headache, proper injection technique for site pain). Always report side effects that are persistent or severe. Never hesitate to call your provider if you are unsure about a symptom. Patient safety is a top priority, and your healthcare team may adjust your dose or suggest supportive care if needed to ensure that side effects are minimized.
Do I need to have any monitoring or tests while on sermorelin?
Yes. Regular monitoring is an important part of sermorelin therapy to ensure it’s working effectively and safely. Your doctor will typically schedule periodic blood tests, the most important being IGF-1 levels. IGF-1 (Insulin-like Growth Factor 1) is a hormone that reflects the amount of growth hormone activity in your body. By measuring IGF-1 during treatment, the doctor can tell if the sermorelin dose is sufficient (IGF-1 should rise into a target range) or if adjustments are needed. IGF-1 also helps guard against giving too high a dose; if it’s above the normal range for your age, the dose might be lowered to avoid side effects.
In addition to IGF-1, your provider might monitor thyroid function tests (since GH and thyroid hormones interact and sermorelin could unveil a thyroid issue). If you have diabetes or prediabetes, blood glucose or HbA1c might be checked to ensure your sugar control remains stable. Children on sermorelin will have their growth (height and weight) tracked carefully over time, and bone age X-rays periodically, to see if they are responding in terms of growth velocity.
Adults might have their body composition (muscle vs fat mass), bone density, or quality-of-life indicators monitored over longer courses of therapy. Furthermore, because antibodies to sermorelin can develop, if there’s any sign that the medication isn’t working as expected (for instance, IGF-1 stops rising despite dose increases), the doctor might investigate if neutralizing antibodies are a factor; though this is uncommon.
Safety labs could include checking your liver and kidney function occasionally, although sermorelin isn’t known to be toxic to those organs. If you have a pituitary condition, periodic MRI scans might be done as part of routine follow-up, but that’s not due to sermorelin itself; rather to monitor the underlying condition.
In summary, you can expect: regular clinic visits and blood tests (IGF-1 and others) every few months initially. As treatment continues and things are stable, the frequency may be reduced (for example, checks every 6 months). It’s very important to adhere to the monitoring plan. This allows your provider to tailor the dose just right for you and to catch any potential issues early. Think of it as part of the therapy; sermorelin is not a one-time fix; it’s a dynamic treatment that’s optimized through ongoing assessment. By staying engaged with your follow-ups, you’ll help ensure the therapy is as safe and beneficial as possible for you.
Can sermorelin be used as an anti-aging or fitness supplement?
No, not legitimately. Sermorelin is a prescription medication intended for specific medical conditions, namely growth hormone deficiency or related diagnoses confirmed by a physician. It is not approved for anti-aging, bodybuilding, or athletic enhancement in individuals with normal growth hormone levels.
While you may come across claims online that sermorelin (and similar peptides) can reverse aging, increase muscle mass, or improve athletic performance, these uses are not backed by robust clinical evidence and are considered off-label and potentially unsafe. In fact, federal regulations prohibit the dispensing of growth hormone for anti-aging or body-building purposes. Using sermorelin without a true medical need won’t turn back the clock; but it could pose risks, like disrupting your hormone balance or causing side effects unnecessarily.
Some short-term studies in healthy older adults have been done with GHRH analogs like sermorelin or other secretagogues, and while they sometimes show increases in lean mass or decreases in fat mass, the improvements are modest and can be accompanied by side effects (e.g. elevated blood sugar or joint pain). Major medical organizations do not endorse growth hormone or its secretagogues for anti-aging. For example, endocrine experts caution that the age-related GH decline might even be protective (there’s ongoing debate), and artificially raising hormone levels in someone without deficiency could do more harm than good.
Additionally, if someone were to misuse sermorelin in hopes of fitness gains, they would still need a prescription and supervision; reputable providers will not prescribe it simply for wellness in a person with normal hormones. The bottom line: sermorelin should only be used if you have a diagnosable growth hormone deficiency or a related medical condition.
If you are an adult looking to increase vitality or muscle, speak with a doctor about proven, safe methods, such as exercise, nutrition, and managing testosterone or other factors if clinically indicated. Sermorelin is not a magic youth serum, and it should not be viewed as a casual supplement. Taking it without need exposes you to needless risks and is not legally or medically sanctioned. Always approach hormone therapies with caution and skepticism towards anti-aging fads. Your health provider can guide you to safer ways to maintain health and fitness that don’t involve off-label use of potent peptides.
Does sermorelin need to be refrigerated, and how should I store it while traveling?
Yes, sermorelin should be kept refrigerated to maintain its stability. Both the dry powder vials (before mixing) and the mixed solution (after reconstitution) are best stored in the refrigerator at 36-46°F (2-8°C). When you have a supply of sermorelin at home, keep it in the fridge in its original packaging to protect it from light. Do not freeze it.
If you are traveling or need to take a dose on the go, use a small cooler or insulated bag with ice packs to keep the medication cold. Generally, sermorelin can stay at room temperature for a limited time (up to 1-2 days maximum for the unmixed powder, and usually less for the mixed solution; check your pharmacy’s guidance) without significant loss of potency, but it’s safer to keep it chilled whenever possible.
If your sermorelin is left unrefrigerated by accident, contact your pharmacy or doctor; if it was at room temperature for only a short while (e.g., a few hours), it is probably fine to continue using, but beyond 72 hours at room temp the medication might degrade and should be replaced.
While traveling, also remember to protect the vial from direct sunlight and heat. Don’t leave it in a hot car or in checked luggage that may be exposed to extreme temperatures. It’s usually recommended to carry it in your hand luggage on a plane (with a doctor’s note, since it involves syringes and vials, to show at security if needed). If you’re staying in a hotel, you can request a refrigerator.
In summary, consistent refrigeration is key. Before using each dose, take the vial out, prepare your injection, and return the vial to the fridge promptly. Proper storage ensures that the sermorelin remains effective for the full duration of your prescription. Always check the expiration or beyond-use date on the vial; do not use it past that date, as peptides can break down even if kept cold.
If you have any doubts about the integrity of the medication (for instance, if it changed color or appearance, or was inadvertently left out), err on the side of caution and consult your healthcare provider or pharmacist for advice. Good storage practices are an important part of therapy with sermorelin, as they help guarantee you’re getting the correct dose potency each time.
IMPORTANT PRESCRIBER & PATIENT NOTICE
This preparation is compounded only when the prescribing practitioner documents that the FDA-approved product does not meet the patient’s medical needs (e.g., different strength, excipient profile, or delivery route).
The formulation described has not been reviewed by the FDA for safety or efficacy.
Scientific literature cited on this page evaluates the active pharmaceutical ingredient; clinical data generated by other entities, are not used to claim or imply equivalence.
Administration Instructions
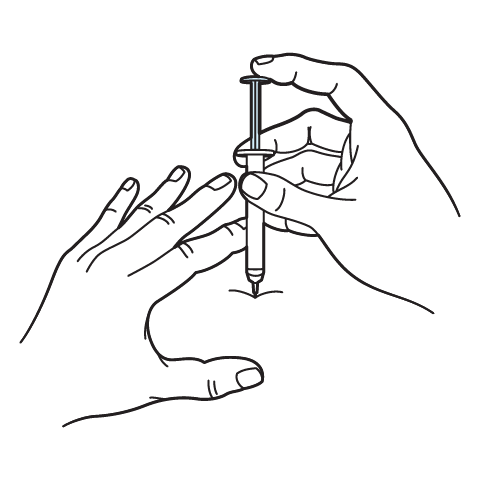
Intramuscular Injection Instructions
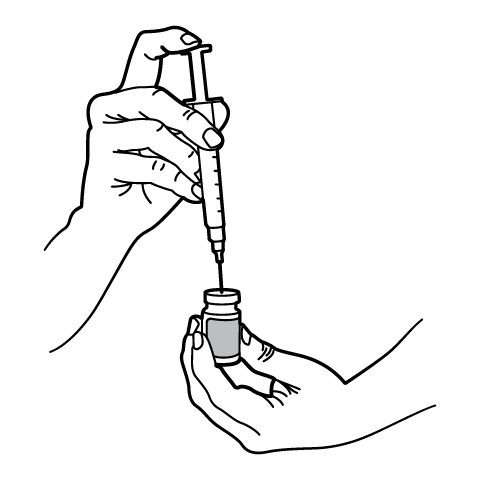
Reconstitution Instructions
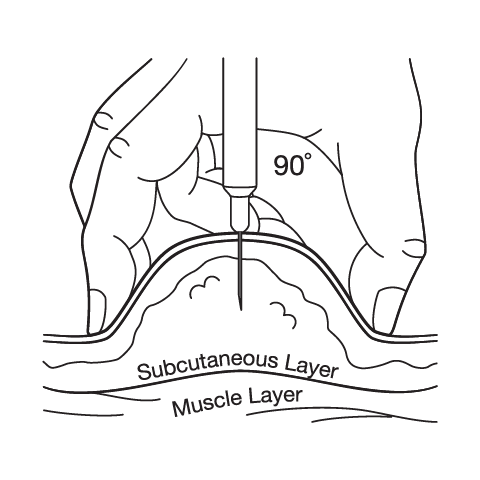
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.




 Sermorelin Acetate ODT
Sermorelin Acetate ODT Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection Testosterone Cypionate Injection
Testosterone Cypionate Injection Anastrozole Capsules
Anastrozole Capsules Enclomiphene Citrate Capsules
Enclomiphene Citrate Capsules Lipo-B Injection
Lipo-B Injection