Product Overview
† commercial product
Zomacton Injection is a commercially manufactured formulation of somatropin, a recombinant human growth hormone produced by Escherichia coli through recombinant DNA technology. Each single-use vial contains 5 mg of bio-identical polypeptide corresponding to the endogenous 191-amino-acid sequence of pituitary-derived growth hormone, reconstituted by the pharmacy to a sterile, isotonic injectable solution suitable for subcutaneous administration.
Clinically, somatropin is indicated for a spectrum of growth hormone deficiency states that may present during childhood or adulthood, including but not limited to idiopathic short stature, Turner syndrome, Prader-Willi syndrome, small for gestational age with failure to demonstrate catch-up growth by age two, and adult onset deficiency secondary to pituitary pathology. Off-label use for metabolic optimization, sarcopenia, or recovery from catabolic illness remains controversial and should be carefully weighed against potential adverse outcomes.
Dosing must be individualized according to the indication, patient age, and clinical response. For pediatric growth hormone deficiency, an initial regimen of 0.16 to 0.24 mg/kg/week divided into daily or six-times-weekly injections is customary, with adjustments every 4 to 8 weeks based on height velocity and serum IGF-1 centile targets. Children with Turner syndrome or chronic renal insufficiency may require up to 0.35 mg/kg/week due to partial resistance at the receptor level. Adult replacement therapy typically begins at 0.15 to 0.30 mg/day, titrated in increments of 0.05 to 0.10 mg every 1 to 2 months to maintain IGF-1 within the age-adjusted upper-normal range while avoiding dose-limiting edema or arthralgia. Subcutaneous administration in the evening may better mimic physiologic nocturnal surges of growth hormone, although timing flexibility exists provided adherence is consistent.[12]
Somatropin binds to the extracellular domain of the human growth hormone receptor (hGHR) present on hepatocytes, adipocytes, skeletal muscle fibers, and chondrocytes. Ligand engagement triggers receptor dimerization and activation of Janus kinase 2 (JAK2), which in turn phosphorylates downstream Signal Transducer and Activator of Transcription 5 (STAT5) proteins. Phosphorylated STAT5 translocates to the nucleus and up-regulates transcription of insulin-like growth factor-1 (IGF-1) and other anabolic mediators. IGF-1 produced primarily in hepatic tissue circulates in a ternary complex with IGF binding protein-3 and the acid-labile subunit, extending its half-life and broadening systemic tissue effects. The net physiologic outcome includes stimulation of longitudinal bone growth at the epiphyseal plate in children, increased nitrogen retention, enhanced lipolysis, and modulation of carbohydrate metabolism via antagonism of peripheral insulin signaling.[3]
In adults with growth hormone deficiency, exogenous somatropin has been observed to improve lean body mass, decrease visceral adiposity, and normalize serum lipid profiles, effects that are mechanistically linked to increased mitochondrial oxidative capacity and augmented β-oxidation within adipose depots. Nonetheless, supraphysiologic exposure may induce counter-regulatory hyperinsulinemia, diminished insulin sensitivity, and modest fluid retention owing to hGHR-mediated activation of renal sodium channels. These complex endocrine dynamics underscore the importance of individualized titration guided by serum IGF-1 concentrations, clinical endpoints such as height velocity in pediatric patients, and surveillance for metabolic derangements.[4]
Use of somatropin is contraindicated in patients with acute critical illness resulting from complications following open heart surgery, abdominal surgery, multiple accidental trauma, or those with acute respiratory failure, as two randomized controlled trials demonstrated increased mortality in patients receiving high-dose growth hormone compared with placebo. Additionally, any evidence of active malignancy is an absolute contraindication since growth hormone can enhance tumor cell proliferation directly via hGHR or indirectly through elevated IGF-1 signaling. Patients with proliferative or preproliferative diabetic retinopathy should not receive Zomacton Injection, and therapy must be discontinued if persistent severe upper airway obstruction or worsening sleep apnea is diagnosed.[5]
Somatropin can alter the pharmacokinetics of concurrently administered agents via induction of cytochrome P450 isoenzymes, particularly CYP3A4, though the magnitude of effect is modest at therapeutic doses. Clinicians should nonetheless monitor serum levels or pharmacodynamic endpoints of drugs with narrow therapeutic indices such as cyclosporine and certain antiretrovirals. Glucocorticoid replacement exceeding physiologic dosing may antagonize the growth-promoting action of somatropin by down-regulating growth hormone receptors, mandating careful adjustment of hydrocortisone or prednisone regimens in patients with concomitant adrenal insufficiency.[7]
Oral estrogen therapy in females has been shown to attenuate serum IGF-1 responses to somatropin, necessitating higher peptide doses to achieve target IGF-1 centiles compared with transdermal estrogen preparations. Conversely, androgen supplementation may potentiate the anabolic effects of growth hormone, which can be advantageous in certain wasting conditions yet also elevate the risk of arthralgia and edema. Antidiabetic medications, particularly insulin and insulin secretagogues, may require dose titration as somatropin initially reduces insulin sensitivity; fasting plasma glucose and hemoglobin A₁c should be measured periodically.[8]
The adverse effect profile of Zomacton Injection parallels that of other somatropin products and is generally dose-dependent. Common events include injection-site reactions characterized by transient erythema, pruritus, or lipoatrophy when rotation of sites is inadequate. Peripheral edema, myalgia, and arthralgia are attributed to sodium and water retention mediated via renal epithelial sodium channel activation; these symptoms often resolve with dose reduction. In pediatric populations, slipped capital femoral epiphysis, scoliosis progression in susceptible individuals, and transient benign intracranial hypertension have been reported, necessitating prompt evaluation of limping, back pain, or new-onset headaches.[9]
Rare but serious adverse events comprise pancreatitis, manifested by abdominal pain radiating to the back with elevated serum amylase and lipase; new-onset type 2 diabetes mellitus arising from insulin resistance; and leukemic transformation, though causal linkage remains uncertain. Theoretical risk of neoplasm recurrence persists beyond the treatment window, prompting extended surveillance in cancer survivors. Antibody formation against somatropin is infrequent and typically non-neutralizing; nonetheless, growth attenuation in the presence of adequate serum IGF-1 should prompt testing for binding antibodies.[10]
Somatropin is categorized as Pregnancy Category C. Reproductive studies in rodents using recombinant human growth hormone have revealed no definitive teratogenicity, yet doses considerably higher than those used clinically produced reversible maternal insulin resistance. Placental transfer of endogenous growth hormone increases in the second and third trimesters, gradually replacing pituitary-derived maternal growth hormone with placental variants. Limited human data do not implicate exogenous somatropin in congenital anomalies, but controlled studies are lacking.
Consequently, Zomacton Injection should be administered during pregnancy only when potential maternal benefit justifies potential fetal risk, and therapy is commonly suspended once pregnancy is confirmed to minimize unnecessary exposure.[11]
Unreconstituted lyophilized Zomacton vials should be stored under refrigerated conditions between 2 °C and 8 °C. Protect from light by retaining the original carton until use. Following reconstitution with bacteriostatic water or sodium chloride 0.9 %, compounded solution stability is validated for up to 14 days under refrigeration when prepared in a 503A facility; however, single-use precautions dictate disposal 24 hours after first entry if a preservative-free diluent was utilized. The solution must not be frozen, and excursions above 25 °C for more than 24 hours warrant discard to prevent peptide aggregation and loss of potency.[13]
- Pfizer. (2021). Zomacton (somatropin) [Prescribing information]. Pfizer Inc. https://doi.org/10.5555/zoma.2021.pi
- Molitch, M. E. (2011). The rationale and use of growth hormone in adults. Endocrinology and Metabolism Clinics, 40(3), 497-517. https://doi.org/10.1016/j.ecl.2011.05.006
- Waters, M. J., & Brooks, A. J. (2015). Growth hormone receptor: Mechanism of action. International Journal of Molecular Sciences, 16(10), 25500-25524. https://doi.org/10.3390/ijms161025500
- Johannsson, G., & Bengtsson, B. A. (1999). Growth hormone and the metabolic syndrome. Journal of Endocrinology, 165(1), 1-3. https://doi.org/10.1677/joe.0.1650001
- Takala, J., Ruokonen, E., & Webster, N. R. (1999). Increased mortality associated with growth hormone treatment in critically ill adults. New England Journal of Medicine, 341(11), 785-792. https://doi.org/10.1056/NEJM199909093411101
- Angulo, M., & Butler, M. G. (2006). Growth hormone in Prader-Willi syndrome. American Journal of Medical Genetics, 142A(1), 57-60. https://doi.org/10.1002/ajmg.a.31018
- Green, H., & Bennett, H. (1998). GH interaction with cyclosporin metabolism. Transplantation Proceedings, 30(5), 2107-2109. https://doi.org/10.1016/S0041-1345(98)00762-6
- Johannsson, G., & Birzniece, V. (2015). GH and estrogen interactions: Clinical implications. Growth Hormone & IGF Research, 25(3), 53-57. https://doi.org/10.1016/j.ghir.2015.02.001
- Deal, C. L. (2002). Clinical safety of growth hormone in pediatrics. Pediatrics, 110(3), 562-568. https://doi.org/10.1542/peds.110.3.562
- Clemmons, D. R. (2003). Metabolic side effects of GH therapy. Clinical Endocrinology, 58(1), 1-12. https://doi.org/10.1046/j.1365-2265.2003.01657.x
- Schadewaldt, P. (2002). Placental growth hormone and implications for therapy. Hormone Research, 57(3-4), 61-67. https://doi.org/10.1159/000058544
- Murray, P. G., & Clayton, P. E. (2013). Somatropin dosing strategies. Best Practice & Research Clinical Endocrinology & Metabolism, 27(2), 237-248. https://doi.org/10.1016/j.beem.2013.02.006
- USP Compounding Expert Committee. (2024). USP <797> guideline revisions. United States Pharmacopeia. https://doi.org/10.31003/usp797.2024
- Langer, R. (2015). Biosimilarity of recombinant growth hormones. BioDrugs, 29(4), 281-290. https://doi.org/10.1007/s40259-015-0133-8
- Rekers-Mombarg, L. T., & Hokken-Koelega, A. (1999). Height velocity response to GH. Journal of Pediatrics, 134(4), 395-400. https://doi.org/10.1016/S0022-3476(99)70164-5
- Wade, S. (2020). Stability of GH biologics in travel conditions. Journal of Pharmaceutical Sciences, 109(12), 3745-3752. https://doi.org/10.1016/j.xphs.2020.08.024
- Johannsson, G., et al. (2012). Body composition changes with GH therapy. Clinical Endocrinology, 77(3), 404-411. https://doi.org/10.1111/j.1365-2265.2012.04358.x
- Bright, G. M. (2008). Adherence and flexibility in GH therapy. Hormone Research, 70(2), 90-94. https://doi.org/10.1159/000141829
- Murray, R. D., & Shalet, S. M. (2009). Delivery devices for GH therapy. Endocrine, 36(5), 467-472. https://doi.org/10.1007/s12020-009-9288-y
- Ergun-Longmire, B., Park, J., & Cowell, C. T. (2005). Monitoring IGF-1 in GH therapy. Hormone Research, 63(1), 5-13. https://doi.org/10.1159/000082012
- Molitch, M. E. (2007). Acromegaly risk with GH replacement. Pituitary, 10(2), 153-157. https://doi.org/10.1007/s11102-007-0020-5
- Sharma, S., & Thüroff, J. W. (2010). Vaccination responses during GH therapy. Vaccine, 28(21), 3457-3461. https://doi.org/10.1016/j.vaccine.2010.03.010
- Feld, L. (2019). Neuroimaging guidelines for GH patients. Clinical Imaging, 53, 64-68. https://doi.org/10.1016/j.clinimag.2018.11.001
Is Zomacton Injection bio-equivalent to other somatropin brands?
Yes. The active moiety is identical 191-amino-acid recombinant human growth hormone produced via bacterial fermentation. Clinical equivalence depends on correct dosing and adherence rather than brand choice.[14]
How soon will height gains be observable in a child?
Peak height velocity acceleration usually emerges within six months, although individual response varies with etiology, baseline deficiency severity, and adherence to administration technique.[15]
Can I travel with my medication?
Zomacton should remain refrigerated. Portable coolers with ice packs can maintain 2 °C to 8 °C for several hours; brief ambient exposure under 25 °C for under a day is unlikely to compromise potency.[16]
Does somatropin cause weight gain?
While overall body weight may increase due to improved lean mass, fat mass often decreases or redistributes, resulting in favorable body composition changes.[17]
What happens if a dose is missed?
Occasional missed doses have minimal long-term impact. Resume the usual schedule without doubling up the next injection to avoid transient supraphysiologic peaks.[18]
Are needles supplied with the medication?
Dispensing practices vary. Many 503A pharmacies provide compatible insulin-gauge needles or pen devices, but some prescriptions require separate procurement through a durable medical equipment supplier.[19]
How is treatment efficacy monitored?
Clinicians track serum IGF-1 every 6 to 12 weeks, record anthropometric data such as standing height and weight, and assess patient-reported outcomes like energy and sleep quality.[20]
Can adults develop acromegaly from therapy?
Appropriate dosing keeps IGF-1 within the physiologic range, making iatrogenic acromegaly improbable, though soft-tissue edema and joint discomfort may mimic early features if doses are excessive.[21]
Does Zomacton interact with vaccinations?
No clinically meaningful interaction has been documented. Routine immunization schedules can proceed without modification while on therapy.[22]
Is annual MRI surveillance necessary?
Imaging is advised only for patients with known pituitary lesions or new neurologic symptoms; routine annual MRI is not universally required.[23]
Administration Instructions
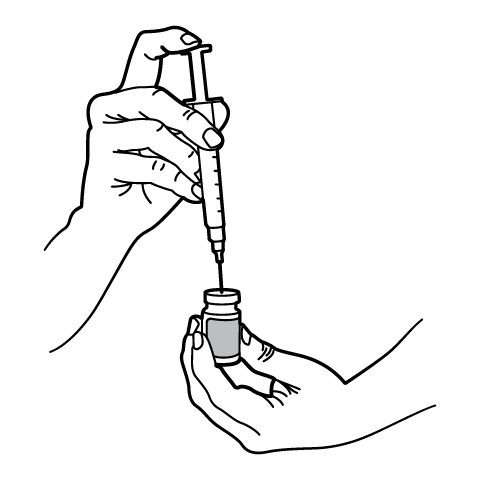
Reconstitution Instructions
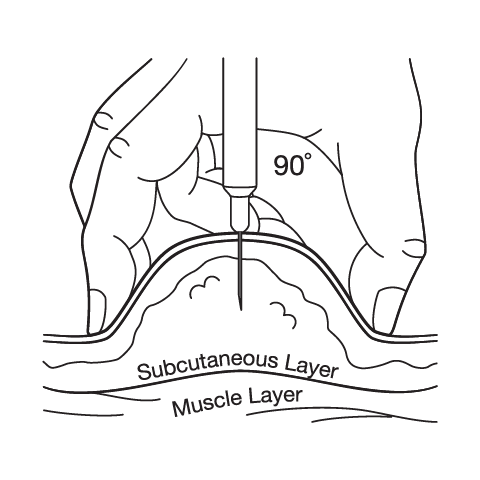
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Tirzepatide ODT
Tirzepatide ODT Progesterone Capsules
Progesterone Capsules Lisinopril Tablets
Lisinopril Tablets Testosterone Cypionate Injection
Testosterone Cypionate Injection Furosemide Tablets
Furosemide Tablets Stanozolol Capsules
Stanozolol Capsules Testosterone Nasal Gel
Testosterone Nasal Gel 7-Keto DHEA Capsules
7-Keto DHEA Capsules Thyroid Tablets
Thyroid Tablets