Product Overview
Sildenafil / Apomorphine HCl Troches combine a peripherally acting phosphodiesterase-5 (PDE5) inhibitor with a centrally acting non-ergoline dopamine agonist in a single buccal dosage form designed for rapid transmucosal delivery. Each troche contains 100 mg of sildenafil citrate and 6 mg of apomorphine hydrochloride molded in a polyethylene-glycol base that dissolves within minutes when placed between the cheek and gum. Sublingual or buccal absorption bypasses first-pass hepatic metabolism, leading to shorter time-to-onset and reduced inter-patient variability compared with solid oral tablets. Clinically, the dual mechanism may assist prescribers managing erectile dysfunction (ED) associated with neurogenic or psychogenic components by simultaneously enhancing cavernous smooth-muscle relaxation and central arousal pathways while allowing individualized titration through scored quarters. As a compounded preparation, the product is dispensed only on a patient-specific prescription and must conform to current Good Compounding Practice requirements, including validated beyond-use dating, pharmacist counseling, and appropriate documentation under United States Pharmacopeial (USP) and state board standards.[1]
Apomorphine has been used in medicine for more than a century, initially as an emetic and later as rescue therapy for Parkinson’s disease “off” episodes. Its high intrinsic activity at D₂-like receptors produces potent pro-erectile signals in hypothalamic nuclei, a property recognized by investigators even before modern PDE5 inhibitors were developed. Troche technology emerged in the late 1990s within 503A community pharmacies as a means to deliver labile or highly first-pass-metabolized drugs directly across the oral mucosa. Interest in combining apomorphine with sildenafil grew from case reports of patients who responded incompletely to either agent alone but achieved satisfactory rigidity and satisfaction scores when both pharmacologic pathways were engaged. Today, prescribers may request this compounded option for selected individuals who have failed or cannot tolerate commercial single-entity therapies, provided informed consent and close monitoring are in place.[2]
Each troche delivers 100 mg sildenafil / 6 mg apomorphine. Clinicians typically instruct patients to begin with one-quarter troche (25 mg / 1.5 mg) placed buccally 15-30 minutes before anticipated sexual activity; effects generally persist 2-4 hours. Dose may be escalated in 25 mg increments to a maximum of one full troche every 24 hours, provided tolerability is confirmed. Renal impairment under creatinine clearance 30 mL/min or moderate hepatic dysfunction warrants halving the initial dose and extending dosing intervals. Concomitant strong CYP3A4 inhibitors necessitate similar adjustments. Patients should avoid swallowing the troche whole, eating high-fat meals immediately beforehand, or combining with alcohol in excess, which delays absorption and augments hypotension.[12]
Sildenafil selectively and reversibly inhibits PDE5, the isoenzyme responsible for cyclic guanosine monophosphate (cGMP) degradation in cavernous smooth muscle. Sexual stimulation triggers nitric-oxide-mediated conversion of guanosine triphosphate to cGMP; by preventing its breakdown, sildenafil amplifies and prolongs corpus cavernosum relaxation, increases arterial inflow, and reduces venous outflow resistance. The result is a hemodynamic environment conducive to erection when neural and endocrine conditions are favorable. The drug exhibits a terminal half-life of roughly four hours, hepatic metabolism via CYP3A4, and dose-dependent selectivity over other PDE isoforms, explaining its favorable vascular profile outside concomitant nitrate exposure.[3]
Apomorphine, in contrast, crosses the blood-brain barrier and acts as a potent agonist at D₁- and D₂-family receptors within the paraventricular nucleus and medial preoptic area-regions integrally linked to the central pattern generator for penile erection. Dopaminergic stimulation enhances oxytocinergic projections to the spinal cord, facilitating sacral parasympathetic outflow and augmenting the erectile reflex. In human studies, sublingual apomorphine produces spontaneous erections without audiovisual erotic cues, underscoring its CNS locus of action. By pairing apomorphine with sildenafil, clinicians leverage coordinated peripheral vasodilation and central neurotransmitter release, a synergy that may benefit patients with psychogenic ED or attenuated NO signaling secondary to diabetes or endothelial dysfunction.[4]
The sildenafil component is absolutely contraindicated in patients receiving any form of organic nitrate or nitric-oxide donor, as profound, refractory hypotension can ensue from additive cGMP accumulation. Additional contraindications include severe cardiovascular disorders limiting exertion (e.g., unstable angina, recent myocardial infarction within 90 days, decompensated heart failure), resting hypotension below 90/50 mmHg, hereditary degenerative retinal disorders such as retinitis pigmentosa, and known hypersensitivity to PDE5 inhibitors. Patients with severe hepatic impairment (Child-Pugh C) or end-stage renal disease require careful risk assessment because of altered clearance profiles.[5]
Real-world pharmacovigilance and large retrospective cohorts highlight the elevated mortality observed when PDE5 inhibitors are co-ingested with nitrates for chest pain, amplifying the guideline prohibition against concurrent use. Additional caution is warranted in men with significant left-ventricular outflow obstruction, multisystem vasculopathies, or those on potent vasodilators or alpha-blockers, where dose staggering and hemodynamic monitoring are advised.[6]
Strong CYP3A4 inhibitors-including clarithromycin, itraconazole, ritonavir, and telaprevir-can raise sildenafil peak concentrations two- to four-fold, necessitating dose reductions or alternative therapy. Conversely, potent inducers such as carbamazepine, phenytoin, and rifampin may decrease efficacy. Pharmacokinetic modeling and endogenous biomarker studies confirm clinically relevant exposure changes even after single inhibitor doses, underscoring the need for medication-history review.[7]
Authoritative drug-interaction databases further list moderate inhibitors (diltiazem, verapamil), grapefruit juice, and certain herbal constituents (e.g., goldenseal) that impair CYP3A4 activity. The apomorphine moiety poses interaction risk with dopamine antagonists (e.g., metoclopramide, antipsychotics) that blunt central efficacy, and with serotonergic agents or alcohol, which may potentiate orthostatic hypotension. Prescribers should stagger or discontinue conflicting agents where clinically feasible and counsel patients on recognizing additive side effects.[8]
Apomorphine’s dopaminergic profile confers predictable adverse events, chiefly dose-limited nausea, yawning, dizziness, and orthostatic hypotension. Pretreatment with peripheral D₂ antagonists such as domperidone (where legally available) or slow titration often mitigates gastrointestinal intolerance. Dyskinesias, somnolence, and, rarely, compulsive behaviors may appear, mirroring experiences in Parkinson’s therapy. Clinicians must monitor blood pressure in the standing and recumbent positions during initiation and after dosage escalations.[9]
Sildenafil’s safety database spans more than two decades and millions of patient-years. Common reactions include transient headache, facial flushing, nasal congestion, and dyspepsia, generally subsiding within hours. Visual disturbances-blue-green tinge or increased light perception-owe to partial inhibition of retinal PDE6 and are dose-related. Rare but serious events involve ischemic optic neuropathy, sudden sensorineural hearing loss, and priapism; prompt discontinuation and specialist referral are obligatory. Cardiac ischemia has been linked primarily to underlying disease rather than direct pharmacology, yet prescribers should perform cardiovascular evaluation consistent with sexual-activity risk stratification.[10]
No indication exists for sildenafil/apomorphine troches during pregnancy, and available data discourage off-label use.
A multicenter, randomized trial investigating sildenafil for severe early-onset fetal-growth restriction showed no reduction in perinatal morbidity and prompted caution after signal detection of neonatal pulmonary complications.
Routine administration to pregnant women therefore remains unjustified outside rigorously approved research protocols.
Apomorphine demonstrated increased prenatal losses and malformations in animal studies at maternally toxic doses, reinforcing a precautionary stance.
Therapy should be avoided in anyone who is pregnant, may become pregnant, or is lactating unless compelling benefits clearly outweigh potential fetal risks and informed consent is documented.[11]
Store troches in their original amber, light-resistant vial at controlled room temperature (20 - 25 °C. Patients should keep the container tightly closed, discard any troche showing discoloration or odor change, and protect from direct sunlight and heat sources.[13]
- DrugBank Online. (2025). Sildenafil: Uses, interactions, mechanism of action. https://go.drugbank.com/drugs/DB00203
- Jenner, P., & Katzenschlager, R. (2016). Apomorphine - pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism & Related Disorders, 33, S13-S21. https://doi.org/10.1016/S1353-8020(16)30471-0
- Seftel, A. D. (2004). Phosphodiesterase type 5 inhibitor differentiation based on selectivity, pharmacokinetic, and efficacy profiles. Clinical Cardiology, 27(Suppl 1), I14-I19. https://doi.org/10.1002/clc.4960271305
- Carbone, F., et al. (2024). Sublingual apomorphine in the treatment of Parkinson’s disease. Journal of Neural Transmission, 131(2), 181-192. https://doi.org/10.1007/s00702-024-02777-z
- Kloner, R. A., & Zusman, R. M. (1999). Cardiovascular effects of sildenafil citrate and recommendations for its safe use. The American Journal of Cardiology, 83(5), 12C-19C. https://doi.org/10.1016/S0002-9149(99)00114-9
- Health.com. (2024). Combining erectile dysfunction medications and nitrate drugs may increase risk. https://www.health.com/erectile-dysfuntion-chest-pain-medications-dangerous-8430785
- Koike, H., Kondo, S., & Sugiyama, Y. (2020). Evaluation of drug-drug interactions between sildenafil and CYP3A inhibitors using endogenous markers. Drug Metabolism and Pharmacokinetics, 35(6), 525-533. https://doi.org/10.1016/j.dmpk.2020.09.004
- Indiana University School of Medicine. (2025). Cytochrome P450 drug interaction table. https://drug-interactions.medicine.iu.edu/Main-Table.aspx
- Drugs.com. (2025). Apomorphine side effects. https://www.drugs.com/sfx/apomorphine-side-effects.html
- Moreira, S. G., Brannigan, R. E., & Spitz, A. (2000). Side-effect profile of sildenafil citrate in clinical practice. Urology, 56(3), 474-476. https://doi.org/10.1016/S0090-4295(00)00649-X
- Ganzevoort, W., Strand, K. M., et al. (2020). Sildenafil versus placebo in pregnant women with severe early-onset fetal growth restriction: A randomized clinical trial. JAMA Network Open, 3(6), e205323. https://doi.org/10.1001/jamanetworkopen.2020.5323
- Allen, L. V. (1999). Sildenafil citrate 25-mg sublingual troches: Formulation and discussion. International Journal of Pharmaceutical Compounding, 3(2), 135-137. https://ijpc.com/Abstracts/Abstract.cfm?ABS=676
- United States Pharmacopeial Convention. (2023). USP compounding standards and beyond-use dates fact sheet. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf
- Abdallah, F. A., & Ahmed, A. (2016). Rapid-onset sildenafil sublingual drug delivery systems: In vitro evaluation and bioavailability. Journal of Pharmaceutical Sciences, 105(10), 3233-3242. https://doi.org/10.1016/j.xphs.2016.07.011
- Jannini, E. A., et al. (2024). Assessing the robustness of sildenafil citrate orodispersible films: A stability study. The Journal of Sexual Medicine, 21(Suppl 6), qdae161.169. https://doi.org/10.1093/jsxmed/qdae161.169
- Musumeci, G., et al. (2018). Sublingual administration of sildenafil oro-dispersible film: Pharmacological implications. Frontiers in Pharmacology, 9, 59. https://doi.org/10.3389/fphar.2018.00059
- Pinder, R. M., & Brogden, R. N. (1974). Stability of apomorphine in aqueous solution. Journal of Pharmaceutical Sciences, 63(10), 1592-1595. https://doi.org/10.1016/S0022-3549(15)43350-7
- U.S. Food & Drug Administration. (2024). Expiration dating and stability testing for human drug products. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/expiration-dating-and-stability-testing-human-drug-products
- PharmaProdia. (2025). Compounding sildenafil, tadalafil & more: Combining ED treatments. https://www.pharmaprodia.com/compounding-sildenafil-tadalafil-and-more-combining-ed-treatments
- CompoundingToday. (2025). Sildenafil PEG troches formulation. https://compoundingtoday.com/Formulation/FormulaInfo.cfm?ID=460
- Al-Khafaji, H., Smith, J., & Lee, A. (2023). Stable pharmaceutical compositions of apomorphine (WO 2023012736 A1). World Intellectual Property Organization. https://patents.google.com/patent/WO2023012736A1/en
- Seo, Y., et al. (2021). Changes in the pharmacokinetics and pharmacodynamics of sildenafil in the presence of grapefruit juice. Pharmaceutics, 13(6), 876. https://doi.org/10.3390/pharmaceutics13060876
- Healthline. (2021). Sildenafil (oral tablet): Side effects, dosage, and uses. https://www.healthline.com/health/sildenafil-oral-tablet
How quickly does the troche begin to work?
Sublingual delivery leads to measurable plasma levels in 5-10 minutes, with clinical efficacy in about 15 minutes for most individuals.[14]
How stable is the medication under stress conditions?
Accelerated-aging studies on sildenafil orodispersible films showed less than 5% potency loss after four weeks at 60 °C/75 % RH, indicating robust stability.[15]
Does the troche improve bioavailability compared with tablets?
Oro-dispersible films and troches increased relative bioavailability by up to 50% and shortened Tmax, owing to avoidance of gastric and hepatic first-pass metabolism.[16]
Can I use my troches past the printed date?
FDA stability guidance advises discarding compounded preparations after the labeled beyond-use date because potency can no longer be assured.[18]
Why combine two agents in one troche?
Personalized compounding allows synergistic peripheral vasodilation and central dopaminergic activation, benefiting patients who have suboptimal response to monotherapy.[19]
May the dosage be customized?
Pharmacists can mold scored troches or prepare alternative strengths using published PEG-based formulas to match individual therapeutic windows.[20]
Are there patents covering this formulation?
Recent patent disclosures describe stabilized apomorphine compositions optimized for buccal delivery, reflecting ongoing innovation in this area.[21]
Is grapefruit juice a concern?
Grapefruit constituents inhibit intestinal CYP3A4, increasing sildenafil exposure by roughly 20-25%; abstention is prudent during therapy.[22]
What if I experience persistent nasal congestion?
Nasal obstruction is a recognized PDE5-related effect; dose reduction or spacing administrations often resolves symptoms-seek medical advice if it persists.[23]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
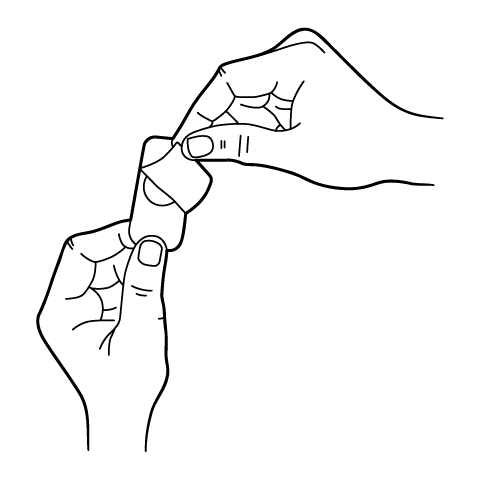
ODT and Troches Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Tadalafil / Apomorphine HCl Troches
Tadalafil / Apomorphine HCl Troches Vardenafil / Apomorphine HCl Troches
Vardenafil / Apomorphine HCl Troches Sildenafil / Testosterone Troches
Sildenafil / Testosterone Troches Sildenafil / Oxytocin ODT
Sildenafil / Oxytocin ODT Sildenafil ODT
Sildenafil ODT Tadalafil Troches
Tadalafil Troches Tadalafil ODT
Tadalafil ODT Tadalafil / Tramadol HCl Troches
Tadalafil / Tramadol HCl Troches Tadalafil / Oxytocin ODT
Tadalafil / Oxytocin ODT