Product Overview
As a 5-alpha reductase inhibitor, Topical Dutasteride prevents the hormone dihydrotestosterone, or DHT, which causes male pattern hair loss, from being produced. In order to determine the safety and efficacy of topical dutasteride for men with male pattern baldness, phase 2 clinical trials are now being conducted in the US.[1] The investigation is expected to be finished in April 2024.
Dutasteride is a 5-alpha-reductase inhibitor (5-ARI) indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) as monotherapy or in combination with tamsulosin. Dutasteride is used off-label for male-pattern baldness (androgenetic alopecia). According to the American Urological Association (AUA) guidelines, 5-ARIs (e.g., dutasteride, finasteride) may be used to prevent the progression of lower urinary tract symptoms (LUTS) secondary to BPH and to reduce the risk of urinary retention and future prostate-related surgery. The guidelines further state that 5-ARIs are appropriate and effective alternatives for men with LUTS secondary to BPH who have demonstrable prostate enlargement. 5-ARIs should not be used in the absence of prostatic enlargement. Dutasteride inhibits both type I and type II 5-alpha-reductase.[2]
Results from two sizable randomized controlled studies suggest that patients using 5-ARIs may have a lower risk of low-grade prostate cancer but a higher risk of high-grade prostate cancer. Therefore, the known treatment benefits of 5-ARIs should be weighed against the potential increased risk of prostate cancer. Patients should be assessed to rule out other urological diseases prior to treatment with dutasteride and periodically thereafter. Because 5-ARIs inhibit the conversion of testosterone to dihydrotestosterone which can cause abnormalities in the external genitalia of the male fetus, pregnant women or women trying to conceive should not handle 5-ARIs since absorption through the skin may result in fetal exposure and severe adverse outcomes.[3][4][5][6][7]
The main androgen that promotes the growth of prostate tissue, 5-alpha-dihydrotestosterone (DHT), is formed when testosterone is converted to dutasteride. This conversion is carried out by the intracellular enzyme 5-alpha-reductase, which has two type I and type II isoforms. The type II isoenzyme is primarily active in reproductive tissues (i.e., prostate, seminal vesicles, epididymides) and is responsible for two-thirds of circulating DHT. The type I isoenzyme is mostly active in the skin and liver. In the treatment of benign prostatic hyperplasia, dutasteride reduces circulating DHT which leads to a reduction in prostate hypertrophy and improvement in urine flow. In male pattern hair loss, the balding scalp contains miniaturized hair follicles and increased amounts of DHT compared with hairy scalp. Dutasteride decreases scalp and serum DHT concentrations, thus interrupting a key factor in the development of androgenetic alopecia in those patients genetically predisposed. Dutasteride does not bind to androgen receptors in humans.
The pharmcodynamic effects of dutasteride include a reduction in total serum prostate-specific antigen (PSA), an increase in total testosterone, an increase in thyroid-stimulating hormone (TSH), and a decrease in total serum DHT. The reduction of serum DHT is dose dependent and is observed within 1 to 2 weeks. Dutasteride does not appear to alter circulating concentrations of sex hormone binding globulin, estradiol, luteinizing hormone, follicle-stimulating hormone, thyroxine (free T4), and dehydroepiandrosterone in healthy volunteers. Dutasteride does not affect the hypothalamic-pituitary-testicular-axis.
Patients who have a history of recognized hypersensitivity to dutasteride (such as angioedema) or any component of the preparation should not take dutasteride. It’s possible for other 5-alpha-reductase inhibitors to cause cross-sensitivity.
The effect of renal impairment on dutasteride pharmacokinetics has not been studied. However, less than 0.1% of a steady-state 0.5 mg dose of dutasteride is recovered in urine, so no adjustment in dosage is expected for patients with renal impairment.
No overall differences in safety or effectiveness have been observed between elderly (> 65 years of age) and other younger adult patients.
Dutasteride is contraindicated for use in females of childbearing potential and is therefore contraindicated during pregnancy. Dutasteride and other 5-alpha-reductase inhibitors inhibit the conversion of testosterone to DHT that can cause abnormalities in the external genitalia of the male fetus. Pregnant women or women trying to conceive should not handle dutasteride capsules because dutasteride is absorbed through the skin and may result in fetal exposure. If skin contact with a leaking capsule occurs, the affected area should be washed with soap and water immediately. Dutasteride is secreted into male semen. The amount of dutasteride in semen that is available for vaginal absorption is estimated to be less than 100 times the concentrations that produces abnormalities in the genitalia of male offspring in animal studies. In addition, dutasteride is more than 96% protein bound in human semen, which may decrease vaginal absorption of the drug.[8]
Dutasteride is not indicated for use in women of childbearing potential and thus is contraindicated for use during breast-feeding. It is not known whether dutasteride is excreted in human milk. Therefore, the effects of dutasteride on infants during breast-feeding cannot be determined.[8]
Dutasteride should be used with caution in patients with hepatic disease; data are limited regarding the incidence of adverse effects or drug accumulation in these patients. The drug is extensively metabolized by the liver and has a half-life of about 5 weeks at steady state. The effect of potent hepatic CYP3A4 isoenzyme inhibitors on the metabolism of dutasteride has not been studied. Care should be taken when administering dutasteride to patients taking potent CYP3A4 inhibitors chronically.
Dutasteride is contraindicated for use in neonates, infants, children, and adolescents. Safety and effectiveness have not been established in these age groups.
Dutasteride reduces total serum prostate specific antigen (PSA) by about 40% after 3 months of treatment and 50% after 6, 12, and 24 months of treatment. This decrease is predictable over the entire range of PSA values, but may vary in individual patients. It is recommended that a new baseline PSA concentration be established after 3-6 months for interpretation of serial PSAs in men taking dutasteride. This new baseline PSA should be used to assess potentially cancer-related changes in PSA. To interpret an isolated PSA value in a man treated with dutasteride for 6 months or more, the PSA value should be doubled for comparison with normal values in untreated men. Increased PSA levels from nadir during dutasteride treatment may indicate possible prostate cancer and should be carefully evaluated regardless of whether or not the levels fall within the normal range for men not taking a 5-alpha reductase inhibitor. The free-to-total PSA ratio (percent free PSA) remains constant at month 12, even under the influence of dutasteride. If clinicians elect to use percent free PSA as an aid in the detection of prostate cancer in men receiving dutasteride, no adjustment to its value appears necessary. In June 2011, a review of two large, randomized controlled trials, the Prostate Cancer Prevention Trial (PCPT) and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial prompted the FDA to alert healthcare professionals of the potential risk of an increased incidence of high-grade prostate cancer in patients receiving finasteride or dutasteride treatment. Results from the REDUCE trial showed that men receiving dutasteride had a 23% decreased risk of being diagnosed with prostate cancer when compared to placebo (p < 0.0001); however, the risk reduction was limited to Gleason score (GS) <= 6 cancers. There was an increased incidence of GS 8-10 prostate cancers with dutasteride compared to placebo (1% vs. 0.5%, respectively).[4] Therefore, in initiating or continuing treatment with dutasteride, clinicians should weigh the known benefits of treatment against the potential risk and be aware that dutasteride may increase the risk of high-grade prostate cancer. Lower urinary tract symptoms of BPH can be indicative of other urological diseases, including prostate cancer. Patients should be assessed to rule out other urological diseases prior to treatment with dutasteride and periodically thereafter. Patients with a large residual urinary volume and/or severely diminished urinary flow may not be good candidates for 5-alpha-reductase inhibitor therapy and should be carefully monitored for urinary tract obstruction.[8]
Males treated with dutasteride should not undergo blood donation until at least 6 months have passed following their last dose. The purpose of this deferred period is to prevent administration of dutasteride to a pregnant female receiving a blood transfusion. Serum levels of dutasteride are detectable for 4-6 months following termination of therapy.
Dutasteride may cause spermatogenesis inhibition or oligospermia, decreased sperm motility, or decreased semen volume. The clinical significance of dutasteride’s effect on semen characteristics for an individual male patient’s fertility is not known; consider the potential effects on semen when assessing a male with infertility. The effects of dutasteride 0.5 mg/day on semen characteristics were evaluated in normal male volunteers aged 18 to 52 (n = 27 dutasteride, n = 23 placebo) throughout 52 weeks of treatment and 24 weeks of post-treatment follow-up. At 52 weeks, the mean percent reductions from baseline in total sperm count, semen volume, and sperm motility were 23%, 26%, and 18%, respectively, in the dutasteride group when adjusted for changes from baseline in the placebo group. Sperm concentration and sperm morphology were unaffected. After 24 weeks of follow-up, the mean percent change in total sperm count in the dutasteride group remained 23% lower than baseline. While mean values for all semen parameters at all time-points remained within the normal ranges and did not meet predefined criteria for a clinically significant change (30%), 2 subjects in the dutasteride group had decreases in sperm count of greater than 90% from baseline at 52 weeks, with partial recovery at the 24-week follow-up.[8][9]
Side effects noted from minimal studies covering topical Dutasteride include:
- Headaches
- Itchiness
- Pain
Research that also included microneedling reported these negative side effects.[10] Therefore, it is unclear which part of the therapy led to these side effects.
Patients may also suffer the following negative effects:
- Dryness and flakiness
- Scaling
- Changes in hair texture or color
- Skin sensitivity
- Greater sensitivity to the sun
Dutasteride is contraindicated for use in females of childbearing potential and is therefore contraindicated during pregnancy. Dutasteride and other 5-alpha-reductase inhibitors inhibit the conversion of testosterone to DHT that can cause abnormalities in the external genitalia of the male fetus. Pregnant women or women trying to conceive should not handle dutasteride capsules because dutasteride is absorbed through the skin and may result in fetal exposure. If skin contact with a leaking capsule occurs, the affected area should be washed with soap and water immediately. Dutasteride is secreted into male semen. The amount of dutasteride in semen that is available for vaginal absorption is estimated to be less than 100 times the concentrations that produces abnormalities in the genitalia of male offspring in animal studies. In addition, dutasteride is more than 96% protein bound in human semen, which may decrease vaginal absorption of the drug.[8]
Dutasteride is not indicated for use in women of childbearing potential and thus is contraindicated for use during breastfeeding. It is not known whether dutasteride is excreted in human milk. Therefore, the effects of dutasteride on infants during breastfeeding cannot be determined.[8]
Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
- National Library of Medicine. (n.d.). Exploratory Clinical Trial of Safety and Efficacy of Daily Application of Topical Dutasteride in Men With Androgenic Alopecia. Clinical Trials.gov. https://clinicaltrials.gov/study/NCT05599243
- Shanshanwal, S. J., & Dhurat, R. S. (2017). Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: A randomized controlled open-label, evaluator-blinded study. Indian journal of dermatology, venereology and leprology, 83(1), 47-54. https://doi.org/10.4103/0378-6323.188652
- Roehrborn C, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Uro
- FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer. Retrieved June 9, 2011. Available on the World Wide Web at: http://www.fda.gov/Drugs/DrugSafety/ucm258314.htm
- McVary KT, Roehrborn CG, Avins AL, et al. American Urological Association guideline: management of benign prostatic hyperplasia (BPH). Linthicum, MD: American Urological Association; 2010. 1-62; Appendix 278-85. Retrieved on February 4, 2020. Available on the Worldwide Web at https://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(bph)-guideline/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014
- Dimitropoulos K, Gravas S. Fixed-dose combination therapy with dutasteride and tamsulosin in the management of benign prostatic hyperplasia. Ther Adv Urol 2016;8:19-28.
- Harcha WG, Martinez JB, Tsai TF, et al. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol 2014;70:489-98.
- Avodart (dutasteride soft gelatin capsules) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2020 Jan.
- Amory JK, Wang C, Swerdloff RS, et al. The effect of 5-alpha-reductaxe inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrin Metab 2007;92(5):1659-65
- Nada, E.A., Sharkawy, R.E., Maged, W.M., & Elmagd, M.A. (2018). Topical dutasteride with microneedling in treatment of male androgenetic alopecia. Southern Medical Journal, 22, 387-400.https://smj.journals.ekb.eg/article_42083_24b61cbba4be9982db23c318414034c0.pdf
Administration Instructions
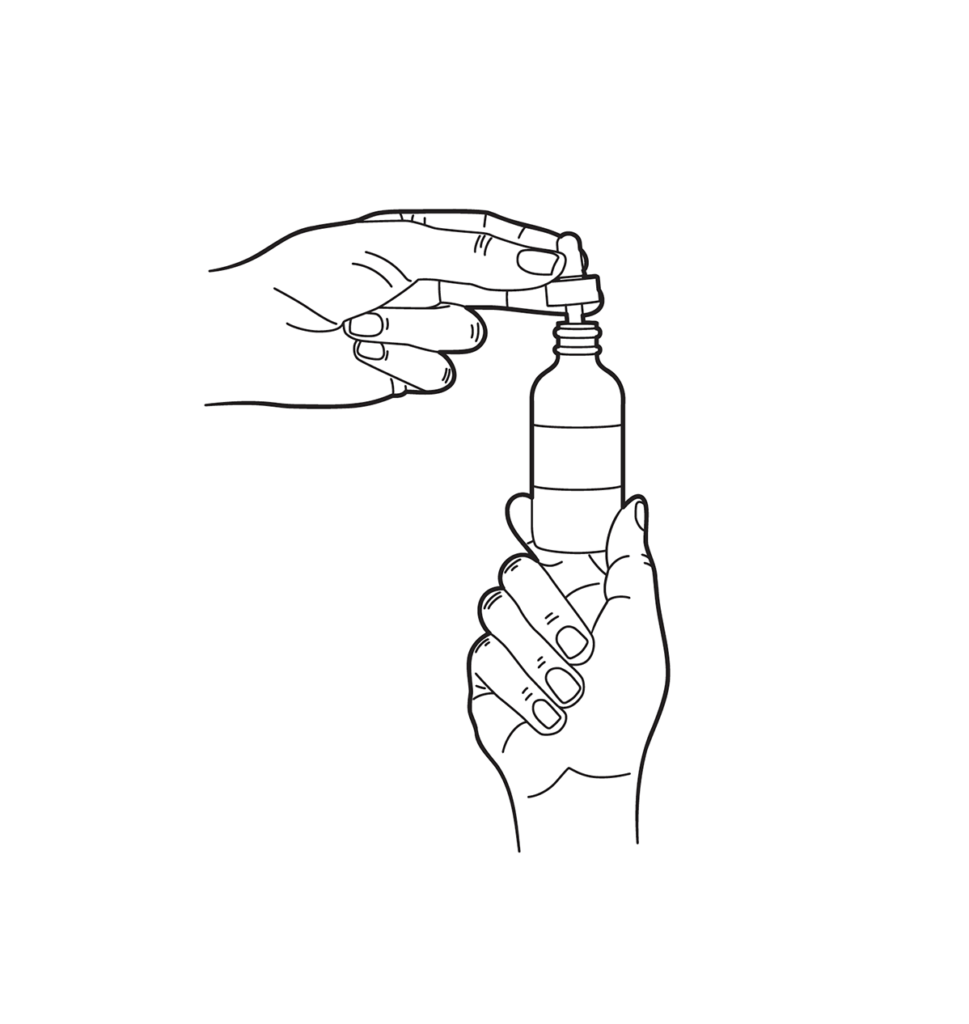
Dropper Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Hair Restore Extreme Scalp Solution
Hair Restore Extreme Scalp Solution Hair Restore Ultra Scalp Solution
Hair Restore Ultra Scalp Solution GHK-Cu Scalp Solution
GHK-Cu Scalp Solution Hair Restore Lite Scalp Solution
Hair Restore Lite Scalp Solution Minoxidil Capsules
Minoxidil Capsules Dutasteride Capsules
Dutasteride Capsules Finasteride Tablets
Finasteride Tablets Biotin / Minoxidil / Spironolactone Capsules
Biotin / Minoxidil / Spironolactone Capsules Biotin / Minoxidil Capsules
Biotin / Minoxidil Capsules Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection