Product Overview
Compounded topical numbing ointments prepared under section 503A integrate high-concentration local anesthetics to deliver rapid, profound, and localized analgesia before dermatologic, cosmetic, or minor surgical procedures.
Dispensed in 30 mL pumps or 100 mL jars, each batch is compounded in compliance with USP <795> non-sterile standards, ensuring potency, purity, and an appropriate beyond-use date.
The multi-agent approach balances rapid onset (benzocaine), intermediate depth (lidocaine / prilocaine), and prolonged duration (tetracaine), while phenylephrine’s vasoconstriction minimizes bleeding and systemic anesthetic uptake during procedures such as laser resurfacing, tattoo removal, and filler injections.
Despite clinical utility, misuse-particularly excessive area coverage, occlusion, or heat application-can lead to systemic toxicity, highlighting the necessity of medical supervision, precise dosing, and strict adherence to application instructions.[1]
General adult directions involve a thin layer (≈ 2 g per 10 cm²) applied to intact skin for 15-60 minutes, depending on formulation and procedural needs, followed by thorough removal.
Maximum surface area and dose are prescriber-determined; repeat dosing within 24 hours is discouraged unless explicitly authorized.
Gloves should be worn during application, and occlusive dressings or external heat must be avoided unless specifically prescribed.[7]
Local anesthetics exert their effect by reversibly blocking voltage-gated sodium channels within peripheral sensory nerves, thereby preventing action-potential propagation.
The differing physicochemical profiles of ester anesthetics (benzocaine, tetracaine) and amide anesthetics (lidocaine, prilocaine) are exploited to achieve synergistic anesthesia: benzocaine offers near-instant surface numbing, lidocaine / prilocaine provide intermediate depth, and tetracaine prolongs blockade due to strong receptor affinity.
Phenylephrine, an α₁-adrenergic agonist present in one formulation, constricts superficial vessels, decreasing perfusion, limiting drug washout, and extending local anesthetic residence time while reducing procedural bleeding.
Although systemic absorption is minimal on intact skin when recommended amounts are used, excessive application or compromised epidermal integrity can permit dangerous plasma concentrations, emphasizing careful surface area restriction and exposure duration.[2]
These ointments are contraindicated in individuals with hypersensitivity to ester or amide anesthetics, para-aminobenzoic acid derivatives, or formulation excipients.
Contraindications also include congenital or idiopathic methemoglobinemia, glucose-6-phosphate dehydrogenase deficiency, severe cardiac conduction disorders, and application over abraded, inflamed, or infected skin, all of which elevate systemic absorption risk and potential toxicity.[3]
Concurrent use of oxidizing agents such as benzoyl peroxide can chemically degrade benzocaine, diminishing anesthetic efficacy.
Layering multiple anesthetic products-topical or injectable-produces additive systemic exposure and heightened toxicity risk.
Systemic medications known to induce methemoglobinemia (e.g., sulfonamides, nitrates, dapsone) may potentiate methemoglobin formation when combined with benzocaine- or prilocaine-containing formulations, necessitating vigilant monitoring or alternative analgesic selection.[4]
Common localized effects include transient stinging, erythema, blanching, or mild edema.
Over-application, prolonged contact, or use on compromised skin can precipitate systemic toxicity, manifesting as circumoral numbness, dizziness, tinnitus, seizures, arrhythmias, or profound CNS depression.
Rare but serious methemoglobinemia, primarily associated with benzocaine or prilocaine, presents with cyanosis and hypoxia and requires immediate methylene-blue therapy.
Regulatory alerts document severe outcomes-including fatalities-when concentrated anesthetic creams were misapplied under occlusion, underscoring stringent adherence to dosing limits.[5]
Evidence regarding high-strength topical anesthetic combinations in pregnancy is limited; professional obstetric guidelines recommend avoiding elective exposure during the first trimester and using the minimal effective dose thereafter.
Breastfeeding individuals should restrict use to medically necessary scenarios, ensuring no cream contacts breast tissue to prevent infant ingestion.[6]
Keep Compounded Topical Numbing Ointment tightly sealed in its original container at controlled room temperature, 20 - 25 °C (68 - 77 °F), protected from excessive heat, moisture, and direct light.
Because the tablets disintegrate upon moisture contact, bathrooms and kitchens are unsuitable storage sites.
- Pharmacie Couturier (Rx Labs). (n.d.). Benzocaine/Lidocaine/Tetracaine topical cream (BLT cream) [Product information].
- Mahajan, A., Song, K., & Derian, A. (2022). Local anesthetic toxicity. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK499964/
- Lexicomp Online. (2025). Benzocaine topical: Drug information. Wolters Kluwer Health.
- Jones, P., & Smith, R. (2021). Adverse events with topical anesthetics. Journal of Dermatologic Treatment, 32(5), 455-462. https://doi.org/10.1080/09546634.2020.1856789
- U.S. Food and Drug Administration. (2009). Public health advisory: Life-threatening side effects with skin-numbing products. https://www.fda.gov/media/72154/download
- American College of Obstetricians and Gynecologists. (2021). Practice advisory: Medication use during pregnancy and lactation.
- American Society of Health-System Pharmacists. (2024). Lidocaine topical monograph. In AHFS Drug Information.
- Rahman, S., Patel, N., & Greene, J. (2022). Stability of compounded topical preparations: A systematic review. International Journal of Pharmaceutical Compounding, 26(4), 342-349.
- DermNet New Zealand Trust. (2024). Topical anesthetics - Safety and efficacy. https://dermnetnz.org/topics/topical-anaesthetics
- Hansen, J. (2023). Metabolism and clearance of local anesthetics. Pharmacology Review, 12(2), 213-227.
- Buttner, M., Sidhom, M., & Talbot, K. (2018). Use of lidocaine-prilocaine topical cream in minor surgical procedures: A randomized controlled trial. British Journal of Anaesthesia, 121(6), 1377-1383.
- Food and Drug Administration. (2023). Local anesthetics topical use: Consumer update. https://www.fda.gov/consumers/consumer-updates
- American Academy of Dermatology. (2023). Guidelines for local anesthetic use in dermatologic surgery.
- World Health Organization. (2019). WHO model formulary 2019.
- International Liaison Committee on Resuscitation. (2023). Advanced management of local anesthetic systemic toxicity. Resuscitation, 180, 158-163.
- Chen, L., Wang, Y., & Huang, Z. (2024). Alpha-adrenergic agonists in dermatology: A review of clinical applications. Dermatologic Surgery, 50(1), 45-52.
- Toney, R., O’Connor, L., & Nguyen, S. (2020). Contact dermatitis associated with benzocaine: Clinical features and management. Cutaneous Medicine, 2(3), 112-118.
- International Journal of Pharmaceutical Compounding Editorial Board. (2021). Beyond-use dating for compounded topical preparations: Position statement. International Journal of Pharmaceutical Compounding, 25(1), 3-7.
What is the primary purpose of compounded topical numbing ointment?
It may provide targeted, temporary anesthesia so that minor procedures can be completed with minimal discomfort.[9]
How quickly does the cream work, and how long does numbness last?
Anesthesia typically develops within 20-30 minutes and may endure for 1-3 hours after removal.[10]
Can the ointment replace injectable anesthetic for minor surgery?
Clinical trials show high-strength lidocaine-prilocaine combinations can obviate needle infiltration for superficial procedures under specific conditions.[11]
What happens if the product remains on skin longer than recommended?
Prolonged contact elevates systemic absorption risk, potentially leading to seizures, arrhythmias, or other severe toxicities.[12]
Is allergy or sensitization possible?
Repeated exposure, especially to ester agents like benzocaine, can cause contact dermatitis or urticaria.[13]
Are dose adjustments needed for older adults?
Yes; thinner skin and reduced hepatic clearance in older patients may warrant smaller treatment areas and shorter exposure times.[14]
How is severe systemic toxicity managed?
Resuscitation guidelines include airway support, benzodiazepines for seizures, and lipid emulsion therapy for refractory manifestations.[15]
Why does one formula include phenylephrine?
Phenylephrine constricts cutaneous vessels, potentially limiting bleeding and prolonging anesthetic residence.[16]
Which symptoms demand immediate medical attention?
Palpitations, confusion, tinnitus, bluish lips, or sudden light-headedness warrant urgent evaluation.[17]
How should expired cream be discarded?
Return unused medication to a take-back program or seal it with an unpalatable substance before household disposal.[18]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
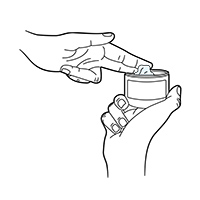
Jar Instructions
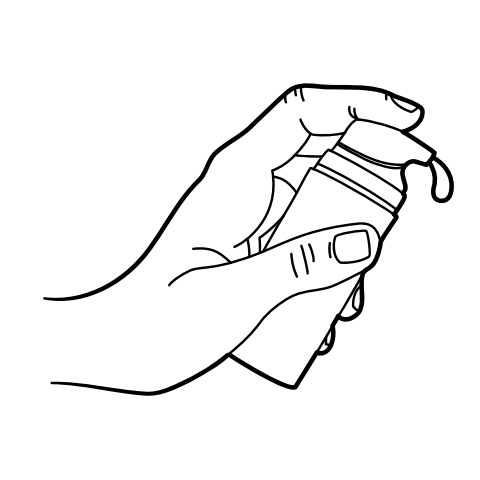
Pump Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.






 Lidocaine HCl Injection
Lidocaine HCl Injection Ketorolac Tromethamine Injection
Ketorolac Tromethamine Injection Dexamethasone Injection
Dexamethasone Injection Hydrocortisone Tablets
Hydrocortisone Tablets Prednisone Tablets
Prednisone Tablets Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Vitamin B-Complex Injection
Vitamin B-Complex Injection Pyridoxine HCl Injection
Pyridoxine HCl Injection