Product Overview
Tirzepatide ODT is a compounded oral disintegrating tablet formulation of tirzepatide that dissolves rapidly on the tongue without the need for water. The medication is available exclusively through our 503A compounding pharmacy, which dispenses each prescription pursuant to a patient-specific prescription following a determination by the prescribing healthcare provider that a commercially available medication is not appropriate for the patient’s treatment. Compounded medications are not FDA-approved medications, and FDA does not review compounded medications for safety or efficacy. Tirzepatide ODT has not undergone clinical trials.
Tirzepatide ODT is available via prescription from a 503A compounding pharmacy and represents a compounded medication tailored for individual patients as determined by their physician. The formulation process involves careful consideration of various factors including dosage precision, stability parameters, and patient-specific considerations that may affect treatment outcomes. Each tablet is formulated to ensure rapid disintegration while maintaining the stability and potency of the active pharmaceutical ingredient throughout its designated beyond-use date.
The oral disintegrating technology employed in this formulation ensures that the medication begins dissolving immediately upon contact with saliva, typically within seconds, making it particularly suitable for physicians to prescribe for patients who have difficulty swallowing traditional tablets or capsules.
The compounding process for Tirzepatide ODT involves sophisticated pharmaceutical techniques to ensure uniform distribution of the active ingredient throughout each tablet while maintaining the delicate balance required for rapid disintegration properties. The formulation incorporates specialized excipients that facilitate quick dissolution while protecting the stability of tirzepatide during storage and handling.
The dosing strategy for Tirzepatide ODT follows a carefully structured titration schedule designed to optimize therapeutic efficacy while minimizing gastrointestinal side effects that commonly occur during treatment initiation. The oral disintegrating tablet should be handled with dry hands and placed immediately on the tongue after removal from its packaging to ensure proper dissolution and absorption.
The timing of dose escalations should be individualized based on patient-specific factors including the severity of their metabolic condition, their treatment goals, and their ability to tolerate the medication’s side effects.
The administration technique for Tirzepatide ODT requires specific attention to ensure optimal drug delivery and absorption. Patients should be instructed to place the tablet on their tongue and allow it to dissolve completely, which typically occurs within thirty to sixty seconds. During this dissolution period, patients should avoid swallowing excess saliva to allow for potential buccal or sublingual absorption of the medication. After the tablet has fully dissolved, patients may swallow normally and may drink water if desired. The medication can be taken at any time of day, with or without food, though maintaining a consistent daily administration time may help establish a routine and improve adherence.
Dose modifications may be necessary in certain clinical situations, as determined by the prescribing healthcare provider. Patients experiencing persistent gastrointestinal side effects that interfere with daily activities or nutritional intake may benefit from extending the duration at each dose level beyond the minimum four weeks before attempting further escalation. Some patients may require eight to twelve weeks at a particular dose before developing adequate tolerance to advance to the next level. In cases where side effects are severe or intolerable, temporary dose reduction may be considered, with subsequent re-escalation attempted more gradually once symptoms have resolved.
Missed dose management for Tirzepatide ODT requires clear patient education to maintain therapeutic consistency while avoiding excessive drug exposure. If a dose is missed, patients should take the missed dose as soon as they remember. Double doses should never be taken to compensate for missed doses, as this could increase the risk of adverse effects without providing additional therapeutic benefit. Patients who frequently miss doses should be counseled about adherence strategies and the importance of consistent medication administration.
Special population dosing considerations may apply to certain patient groups, though specific recommendations for the oral disintegrating tablet formulation may differ from those established for injectable tirzepatide. Elderly patients, generally defined as those sixty-five years and older, do not require routine dose adjustments based solely on age, though they may be more sensitive to the medication’s effects and may benefit from slower titration schedules. The increased prevalence of comorbidities and polypharmacy in elderly patients necessitates careful monitoring during dose escalation. Renal function should be assessed before initiating therapy and monitored periodically, as age-related decline in kidney function may affect drug elimination and increase susceptibility to adverse effects.
Concurrent insulin therapy requires particular attention to dosing adjustments to prevent hypoglycemia. When initiating Tirzepatide ODT in patients already receiving insulin, the insulin dose should typically be reduced by twenty to thirty percent, with subsequent adjustments based on blood glucose monitoring results. The degree of insulin dose reduction may vary depending on the patient’s baseline glycemic control, insulin sensitivity, and the type of insulin regimen being used. Patients on intensive insulin regimens with multiple daily injections may require more complex adjustments than those on simpler basal insulin regimens.
Tirzepatide ODT has not undergone clinical trials or been reviewed by FDA for safety or efficacy. Tirzepatide ODT may have similar contraindications to injectable tirzepatide, which are discussed below.
Healthcare providers must carefully consider before prescribing this medication to ensure patient safety and optimal therapeutic outcomes. The primary contraindication for tirzepatide involves patients with a personal or family history of medullary thyroid carcinoma or patients with Multiple Endocrine Neoplasia syndrome type 2. This contraindication stems from observations in rodent studies where tirzepatide and similar GLP-1 receptor agonists caused thyroid C-cell tumors at clinically relevant exposures. While the relevance of these findings to humans remains uncertain, the potential risk necessitates avoiding tirzepatide in patients with known susceptibility to these rare thyroid malignancies. Healthcare providers should thoroughly review family medical histories and consider thyroid screening in patients with suspicious symptoms or findings before initiating therapy.
Patients with known hypersensitivity to tirzepatide or any component of the oral disintegrating tablet formulation should not receive this medication. Hypersensitivity reactions may manifest as urticaria, angioedema, bronchospasm, or anaphylaxis, and any previous allergic reaction to tirzepatide or related GLP-1 receptor agonists should be considered an absolute contraindication. The excipients used in the oral disintegrating tablet formulation may differ from those in injectable preparations, so patients should be evaluated for potential sensitivities to these specific components as well. Cross-reactivity between different incretin-based therapies has been reported, suggesting that patients with allergies to other GLP-1 receptor agonists should be approached with extreme caution if tirzepatide therapy is being considered.
Severe gastrointestinal disease represents another important contraindication for tirzepatide, particularly in patients with gastroparesis or other conditions causing significant delays in gastric emptying. Since tirzepatide inherently slows gastric emptying as part of its mechanism of action, administering it to patients with pre-existing gastroparesis could potentially worsen their condition and lead to severe gastrointestinal complications. Patients with inflammatory bowel disease, severe gastroesophageal reflux disease, or other significant gastrointestinal disorders may experience exacerbation of their symptoms with tirzepatide therapy. The oral disintegrating tablet formulation may present unique considerations for patients with oral mucosal conditions or swallowing disorders that could affect proper administration or absorption of the medication.
A history of pancreatitis requires careful evaluation before considering tirzepatide therapy. While the causal relationship between incretin-based therapies and pancreatitis remains debated, postmarketing reports have documented cases of acute pancreatitis in patients receiving GLP-1 receptor agonists. Patients with a history of pancreatitis, particularly those with chronic pancreatitis or recurrent acute episodes, may be at increased risk for pancreatitis recurrence or exacerbation. Additional risk factors for pancreatitis, such as severe hypertriglyceridemia, alcoholism, or cholelithiasis, should be addressed and managed appropriately before initiating tirzepatide therapy. Regular monitoring for signs and symptoms of pancreatitis is essential, and the medication should be discontinued immediately if pancreatitis is suspected.
Pregnancy represents an absolute contraindication for tirzepatide due to potential risks to fetal development. Animal reproduction studies have shown adverse effects on embryo-fetal development, and there are no adequate and well-controlled studies in pregnant women. Women of childbearing potential should use effective contraception during treatment with tirzepatide and should discontinue the medication before planned pregnancy. The long half-life of tirzepatide means that the drug may remain in the system for several weeks after discontinuation, which should be considered when counseling patients about pregnancy planning. The oral disintegrating tablet formulation does not alter these reproductive considerations, and the same precautions apply regardless of the route of administration.
Diabetic retinopathy complications have been reported with rapid improvements in glycemic control, and patients with a history of diabetic retinopathy should be monitored carefully when initiating tirzepatide. While not an absolute contraindication, the presence of proliferative diabetic retinopathy or recent retinal hemorrhage may necessitate ophthalmologic evaluation and treatment before starting tirzepatide therapy. The potential for rapid glucose lowering with tirzepatide could theoretically worsen existing retinopathy, particularly in patients with longstanding poor glycemic control who experience sudden improvement.
Tirzepatide ODT has not undergone clinical trials or been reviewed by FDA for safety or efficacy. Tirzepatide ODT may have similar contraindications to injectable tirzepatide. Therefore, we provide tirzepatide’s interaction considerations below.
Tirzepatide exhibits several clinically significant drug interactions that healthcare providers must carefully consider when prescribing this medication to ensure therapeutic efficacy and patient safety. The primary interaction concern with tirzepatide involves its effect on gastric emptying, which can significantly influence the absorption kinetics of concomitantly administered oral medications. Since tirzepatide delays gastric emptying as part of its therapeutic mechanism, medications that require specific timing relative to meals or those with narrow therapeutic windows may have altered absorption profiles when taken concurrently with tirzepatide. This interaction is particularly relevant during the initial weeks of therapy when the gastric emptying effects are most pronounced, though some degree of delayed emptying persists throughout treatment.
Oral medications that require rapid absorption for acute symptom relief may have reduced effectiveness when administered with tirzepatide. Pain medications, particularly those used for acute pain management, may demonstrate delayed onset of action due to slower gastric emptying. Patients requiring immediate pain relief should be counseled about this potential delay and may need to adjust the timing of their analgesic doses or consider alternative formulations such as sublingual or transdermal preparations when appropriate. Similarly, medications used for acute conditions such as migraine treatments or anti-nausea medications may require dose timing adjustments to ensure optimal therapeutic response.
The interaction between tirzepatide and insulin or insulin secretagogues represents a critical safety consideration that requires careful management. When tirzepatide is added to existing insulin therapy, the risk of hypoglycemia increases substantially, particularly during the initial titration period. The glucose-lowering effects of tirzepatide combined with insulin’s action can result in excessive reduction in blood glucose levels. Healthcare providers typically need to reduce insulin doses by approximately twenty to thirty percent when initiating tirzepatide therapy, with further adjustments based on glucose monitoring results. Sulfonylureas and meglitinides, which stimulate insulin secretion independently of glucose levels, also carry increased hypoglycemia risk when combined with tirzepatide and often require dose reduction or discontinuation.
Oral contraceptives present a unique interaction consideration with tirzepatide due to the potential for reduced absorption and efficacy. The delayed gastric emptying caused by tirzepatide may affect the bioavailability of oral contraceptive hormones, potentially compromising contraceptive efficacy. Women using oral contraceptives should be counseled about this interaction and may need to consider additional barrier methods of contraception, particularly during the first month of tirzepatide therapy or following dose increases. Alternative contraceptive methods such as intrauterine devices, implants, or depot injections that bypass gastrointestinal absorption may be preferable for women requiring reliable contraception while on tirzepatide therapy.
Medications with narrow therapeutic indices require special attention when prescribed alongside tirzepatide. Warfarin and other oral anticoagulants may have altered absorption and potentially variable anticoagulation effects when gastric emptying is delayed. More frequent monitoring of international normalized ratio values may be necessary, particularly during tirzepatide initiation or dose adjustments. Digoxin, lithium, and certain antiepileptic drugs also fall into this category and may require therapeutic drug monitoring to ensure levels remain within the therapeutic range. The oral disintegrating tablet formulation of tirzepatide does not eliminate these concerns, as the systemic effects on gastric emptying occur regardless of the route of administration.
Thyroid hormone replacement therapy interactions with tirzepatide deserve particular consideration given that many patients with metabolic disorders also have concurrent thyroid conditions. Levothyroxine absorption is particularly sensitive to gastric pH and timing relative to meals, and the delayed gastric emptying from tirzepatide may reduce levothyroxine bioavailability. Patients should be advised to take levothyroxine at least thirty to sixty minutes before the first meal of the day and to maintain consistent timing relative to tirzepatide administration. Thyroid function tests should be monitored more frequently during the initial months of combined therapy to ensure adequate thyroid hormone replacement.
Gastrointestinal medications may interact with tirzepatide through various mechanisms. Proton pump inhibitors and H2 receptor antagonists, commonly used for acid reflux management, may be needed more frequently in patients experiencing gastrointestinal side effects from tirzepatide. However, these medications can also affect the absorption of other drugs and nutrients, creating complex interaction scenarios. Metoclopramide and other prokinetic agents that accelerate gastric emptying may theoretically counteract some of tirzepatide’s therapeutic effects and should generally be avoided unless specifically indicated for severe gastroparesis symptoms.
The interaction between tirzepatide and alcohol requires careful patient counseling. Alcohol can independently affect blood glucose levels and may mask hypoglycemia symptoms, creating additional risk when combined with tirzepatide therapy. Furthermore, alcohol may exacerbate gastrointestinal side effects such as nausea and vomiting that are commonly associated with tirzepatide. Patients should be advised to limit alcohol consumption and to be particularly cautious about hypoglycemia risk if they choose to drink alcohol while on tirzepatide therapy.
Herbal supplements and over-the-counter medications may also interact with tirzepatide in clinically relevant ways. Supplements that affect blood glucose levels, such as chromium, alpha-lipoic acid, or bitter melon, may enhance the glucose-lowering effects of tirzepatide and increase hypoglycemia risk. Weight loss supplements, particularly those containing stimulants or compounds that affect appetite, should be used cautiously as they may exacerbate gastrointestinal side effects or create unpredictable metabolic effects when combined with tirzepatide.
Tirzepatide ODT has not undergone clinical trials or been reviewed by FDA for safety or efficacy. Tirzepatide ODT may have similar side effects to injectable tirzepatide, which are discussed below.
The side effect profile of tirzepatide encompasses a range of adverse reactions that vary in frequency and severity, with gastrointestinal effects representing the most commonly reported category of adverse events. Nausea occurs in a substantial proportion of patients initiating tirzepatide therapy, typically affecting thirty to forty percent of individuals during the initial weeks of treatment. This nausea tends to be mild to moderate in severity and often improves with continued use as tolerance develops. The sensation may be most pronounced following meals or during dose escalation periods, and some patients find that eating smaller, more frequent meals helps minimize this discomfort. The oral disintegrating tablet formulation may potentially influence the onset or severity of nausea compared to injectable forms, though individual responses vary considerably.
Vomiting represents another frequent gastrointestinal side effect, occurring in approximately fifteen to twenty percent of patients receiving tirzepatide therapy. Episodes of vomiting are most common during the initiation phase and following dose increases, with frequency and severity typically decreasing over time. Persistent vomiting that leads to dehydration or electrolyte imbalances requires medical attention and may necessitate dose reduction or temporary discontinuation of the medication. The relationship between vomiting episodes and meal timing or composition should be evaluated to identify potential triggers and develop management strategies.
Diarrhea affects approximately twelve to twenty percent of patients using tirzepatide and can range from mild loose stools to more frequent watery bowel movements. This side effect may occur intermittently throughout treatment but is most common during the first few weeks of therapy. The mechanism likely involves the medication’s effects on gastrointestinal motility and secretion patterns. Patients experiencing persistent diarrhea should be monitored for signs of dehydration and electrolyte disturbances, particularly potassium and magnesium depletion. Dietary modifications, such as avoiding high-fat foods or lactose-containing products, may help manage this side effect.
Constipation, though less common than diarrhea, affects approximately six to ten percent of patients and may alternate with periods of loose stools in some individuals. The delayed gastric emptying and altered intestinal motility associated with tirzepatide can contribute to constipation, which may be exacerbated by inadequate fluid intake or dietary fiber. Patients should be counseled about maintaining adequate hydration and fiber intake, and mild laxatives may be considered if constipation becomes problematic. The oral disintegrating tablet formulation should be accompanied by adequate fluid intake throughout the day to help prevent constipation.
Decreased appetite, while often considered a therapeutic effect for weight management, can become problematic if it leads to inadequate nutritional intake or unintended excessive weight loss. This effect occurs in approximately ten to fifteen percent of patients and may be more pronounced during the initial weeks of therapy or following dose escalations. Patients should be monitored for signs of malnutrition, particularly protein deficiency, and may benefit from nutritional counseling to ensure adequate intake of essential nutrients despite reduced appetite. The timing of tirzepatide administration relative to meals may influence the degree of appetite suppression experienced.
Injection site reactions, while not applicable to the oral disintegrating tablet formulation, may be replaced by oral mucosal irritation or taste disturbances specific to the ODT form. Some patients may experience a temporary alteration in taste perception or mild irritation of the oral mucosa where the tablet dissolves. These local effects are generally mild and transient, resolving within minutes to hours after administration. Patients with sensitive oral tissues or existing oral conditions may be more susceptible to these local effects.
Hypoglycemia risk varies significantly depending on concurrent diabetes medications and individual patient factors. When used as monotherapy in patients without diabetes, clinically significant hypoglycemia is rare, occurring in less than one percent of patients. However, when combined with insulin or sulfonylureas, the risk increases substantially, potentially affecting twenty to thirty percent of patients if dose adjustments are not made appropriately. Symptoms of hypoglycemia may include shakiness, sweating, confusion, irritability, rapid heartbeat, and hunger. Patients should be educated about recognizing and treating hypoglycemia, including having rapid-acting glucose sources readily available.
Cardiovascular side effects, though generally mild, may include transient increases in heart rate of approximately two to four beats per minute on average. Some patients may experience palpitations or awareness of their heartbeat, particularly during the initial weeks of therapy. Blood pressure effects are variable, with many patients experiencing modest reductions in blood pressure related to weight loss, while others may have no significant change. Patients with pre-existing cardiovascular conditions should be monitored more closely during tirzepatide initiation and dose adjustments.
Gallbladder-related adverse events, including cholelithiasis and cholecystitis, have been reported with tirzepatide therapy, occurring in approximately one to two percent of patients. The risk appears to be related to rapid weight loss and altered bile composition associated with GLP-1 receptor agonist therapy. Patients should be informed about the signs and symptoms of gallbladder disease, including right upper quadrant pain, particularly after fatty meals, and should seek medical evaluation if these symptoms occur.
Renal effects are primarily related to volume depletion from gastrointestinal side effects rather than direct nephrotoxicity. Acute kidney injury has been reported in patients experiencing severe vomiting or diarrhea leading to dehydration. Patients with pre-existing renal impairment or those taking medications that affect renal function may be at higher risk. Regular monitoring of renal function and maintaining adequate hydration are important preventive measures.
Pancreatitis, though rare, represents a serious potential adverse effect that has been reported with GLP-1 receptor agonist therapy. The incidence with tirzepatide appears to be less than one percent, but patients should be educated about the signs and symptoms of pancreatitis, including severe abdominal pain that may radiate to the back, often accompanied by nausea and vomiting. Any suspicion of pancreatitis warrants immediate medical evaluation and discontinuation of tirzepatide pending investigation.
Allergic reactions ranging from mild rash to severe hypersensitivity have been reported rarely with tirzepatide therapy. Symptoms may include urticaria, pruritus, facial edema, or in severe cases, anaphylaxis. The oral disintegrating tablet formulation may contain different excipients than injectable forms, potentially presenting different allergen profiles. Patients with known allergies should have their sensitivities carefully reviewed before initiating therapy.
If pregnancy occurs while using this medication, you should immediately contact your healthcare provider to discuss whether you should continue using this compounded medication.
You should follow your healthcare providers’ instructions regarding when this medication can be resumed, if discontinued, and whether this medication may be used during breastfeeding.
Proper storage of Tirzepatide ODT is essential for maintaining the medication’s stability and potency throughout its designated beyond-use date, with specific requirements that must be carefully followed to ensure optimal therapeutic efficacy. The oral disintegrating tablets should be stored at controlled room temperature between sixty-eight and seventy-seven degrees Fahrenheit or twenty to twenty-five degrees Celsius, with allowable excursions between fifty-nine and eighty-six degrees Fahrenheit or fifteen to thirty degrees Celsius for brief periods such as during transport or temporary environmental fluctuations. These temperature parameters are critical because exposure to temperatures outside this range can potentially affect the stability of tirzepatide and the integrity of the oral disintegrating tablet matrix, potentially leading to degradation of the active ingredient or changes in the dissolution characteristics of the formulation.
Protection from moisture represents a particularly critical storage requirement for Tirzepatide ODT due to the hygroscopic nature of oral disintegrating tablet formulations. The tablets must be kept in their original packaging until immediately before use, as the specialized packaging materials are designed to provide an effective moisture barrier that prevents premature dissolution or degradation of the tablets. Exposure to atmospheric humidity can cause the tablets to soften, stick together, or begin dissolving prematurely, which would compromise their effectiveness and make proper administration impossible. Patients should be instructed never to transfer the tablets to other containers such as pill organizers or weekly medication dispensers, as these typically do not provide adequate moisture protection.
Light protection is another important storage consideration for Tirzepatide ODT, as exposure to direct sunlight or intense artificial light can potentially accelerate the degradation of tirzepatide and affect the stability of the formulation. The original packaging is designed to provide appropriate light protection, and the medication should be stored in a dark location such as a closed cabinet or drawer rather than on countertops or window sills where it might be exposed to direct light. Prolonged exposure to ultraviolet radiation from sunlight can cause photodegradation of the active pharmaceutical ingredient, potentially reducing potency and creating degradation products that could affect safety or efficacy.
The storage location should be carefully selected to ensure both appropriate environmental conditions and safety from unauthorized access. A bedroom dresser drawer, hallway linen closet, or kitchen cabinet away from heat and moisture sources typically provides suitable storage conditions. Areas to avoid include bathrooms where humidity levels fluctuate significantly, kitchens near stoves or dishwashers that generate heat and steam, vehicles where temperature extremes are common, and locations near windows where temperature and light exposure vary throughout the day. The storage area should also be secure from access by children, pets, or anyone for whom the medication is not prescribed.
Keeping Tirzepatide ODT out of reach of children is a critical safety requirement that extends beyond simple elevation of storage location. The medication should be stored in a locked cabinet or container when possible, particularly in households with young children or when children visit regularly. The oral disintegrating tablet formulation may be particularly appealing to children due to its quick-dissolving nature and potential flavoring agents, making secure storage even more important. Child-resistant packaging should always be properly re-secured after each use, though it should be remembered that child-resistant does not mean child-proof, and additional precautions are necessary.
The beyond-use date indicated on the Tirzepatide ODT packaging represents the date beyond which the medication should not be used, as established by stability testing under specified storage conditions. This date is determined by the compounding pharmacy based on stability data for the specific formulation and should be strictly observed. Patients should be instructed to check the beyond-use date before each administration and to properly dispose of any medication that has exceeded this date, even if it appears unchanged. The beyond-use date assumes proper storage conditions have been maintained throughout the medication’s life, and any significant deviation from recommended storage conditions may necessitate earlier disposal.
Travel considerations for Tirzepatide ODT storage require advance planning to maintain appropriate conditions while away from home. When traveling by air, the medication should be kept in carry-on luggage to avoid temperature extremes in cargo holds and to ensure continuous access to the medication. The original pharmacy packaging with the prescription label should always accompany the medication during travel for security screening and legal purposes. For extended travel, patients should consider the climate of their destination and may need to take additional precautions such as insulated medication bags or portable cooling devices if traveling to very hot climates, though freezing must be avoided.
Temperature excursion management is important for maintaining medication integrity when temporary deviations from recommended storage conditions occur. Brief exposures to temperatures slightly outside the recommended range, such as during transport from the pharmacy or short power outages, typically do not compromise medication stability if the duration is limited and the temperature deviation is moderate. However, prolonged exposure to extreme temperatures, particularly heat above ninety degrees Fahrenheit or freezing conditions, may irreversibly damage the medication. If patients suspect their medication has been exposed to extreme temperatures, they should contact their pharmacist or healthcare provider for guidance rather than continuing to use potentially compromised medication.
Proper disposal of unused or expired Tirzepatide ODT requires environmentally responsible methods that prevent unauthorized access and minimize environmental impact. Patients should not flush tablets down the toilet or pour them into drains unless specifically instructed to do so, as this can contribute to pharmaceutical contamination of water supplies. Many communities offer drug take-back programs or events where medications can be safely disposed of, and these represent the preferred disposal method when available. If take-back programs are not accessible, the FDA recommends mixing unused tablets with an unpalatable substance such as used coffee grounds or cat litter, sealing the mixture in a container, and disposing of it in household trash after removing or obscuring personal information from the prescription label.
Quality indicators that patients should monitor include the physical appearance and characteristics of the tablets, though visual inspection should not replace adherence to beyond-use dating. Any tablets that appear discolored, broken, or partially dissolved should not be used and may indicate exposure to inappropriate storage conditions. Changes in tablet texture, such as becoming soft or sticky, suggest moisture exposure and compromise of the formulation. Patients should be instructed to report any concerns about medication appearance or unusual effects to their pharmacist or healthcare provider promptly.
What makes Tirzepatide ODT different from regular tirzepatide injections?
Tirzepatide ODT is an oral disintegrating tablet formulation that dissolves rapidly on the tongue without requiring water. Physicians may determine this compounded medication is necessary for patients who have needle phobia, difficulty with manual dexterity required for injections, or lifestyle factors that make regular injections inconvenient. The formulation is specially compounded by 503A pharmacies to meet individual patient needs as determined by the prescribing physician, allowing the prescribing physician to customize dosing and potentially other aspects of the formulation based on specific a patient’s needs. Compounded medications are not FDA-approved drugs. FDA does not review compounded medications for safety or efficacy.
How quickly does Tirzepatide ODT start working?
Patients should discuss with their physicians regarding expected outcomes and timing.
Can Tirzepatide ODT be taken with other diabetes medications?
Tirzepatide ODT can be combined with various other diabetes medications, though specific combinations require careful consideration and potential dose adjustments to optimize safety and efficacy. Metformin is commonly continued when starting tirzepatide, as the combination provides complementary mechanisms of action without significantly increasing hypoglycemia risk. When combined with insulin or sulfonylureas, dose reductions of these medications are typically necessary to prevent hypoglycemia, with adjustments based on blood glucose monitoring results. SGLT2 inhibitors may be used concurrently with tirzepatide, though patients should be monitored for volume depletion effects. DPP-4 inhibitors are generally discontinued when starting tirzepatide since both medications work through incretin pathways, and combination use is not recommended. Each patient’s medication regimen should be individually assessed and adjusted by their healthcare provider based on their specific clinical situation.
What should I do if I experience persistent nausea with Tirzepatide ODT?
Managing nausea associated with Tirzepatide ODT involves several strategies that can significantly improve comfort and tolerance. Eating smaller, more frequent meals throughout the day rather than large meals can help reduce nausea severity. Avoiding foods that are high in fat, spicy, or have strong odors may also help, as these can exacerbate gastrointestinal discomfort. Staying well-hydrated with small, frequent sips of water or clear liquids can prevent dehydration and may reduce nausea. Some patients find that taking the medication at a different time of day, such as before bedtime, allows them to sleep through the peak nausea period. If nausea persists despite these measures, healthcare providers may recommend extending the time at the current dose before attempting further dose escalation, or in some cases, temporarily reducing the dose. Anti-nausea medications may be prescribed for short-term use during the adjustment period, though most patients find that nausea improves significantly after the first few weeks of treatment.
How long do I need to stay on Tirzepatide ODT?
The duration of Tirzepatide ODT therapy will be determined by your prescribing physician and depends on individual treatment goals, response to therapy, and ongoing clinical needs, with many patients requiring long-term or indefinite treatment to maintain therapeutic benefits . Patients using tirzepatide primarily for weight management should understand that weight regain is common after discontinuation, and long-term therapy may be necessary to maintain weight loss. The decision to continue or discontinue therapy should be made in consultation with healthcare providers based on regular assessments of benefits versus risks, achievement of treatment goals, and individual patient preferences. Some patients may successfully transition to other maintenance strategies after achieving their goals with tirzepatide, while others may benefit from continued long-term therapy.
Can I drink alcohol while taking Tirzepatide ODT?
Alcohol consumption while taking Tirzepatide ODT requires careful consideration and moderation due to several potential interactions and risks. Alcohol can affect blood glucose levels unpredictably, potentially causing hypoglycemia, especially in patients taking other glucose-lowering medications along with tirzepatide. The consumption of alcohol may also exacerbate gastrointestinal side effects such as nausea and vomiting that are common with tirzepatide therapy. Additionally, alcohol can mask the symptoms of hypoglycemia, making it harder to recognize and treat low blood sugar episodes promptly. If patients choose to drink alcohol, they should do so in moderation, with food, and should monitor their blood glucose levels more frequently. It is advisable to discuss alcohol consumption with healthcare providers to understand individual risks and establish safe consumption guidelines.
What happens if I miss a dose of Tirzepatide ODT?
When a dose of Tirzepatide ODT is missed, the appropriate action depends on how much time has elapsed since the scheduled dose. If remembered within three days of the missed dose, patients should take the missed dose as soon as possible and then resume their regular dosing schedule. If more than three days have passed since the missed dose, patients should skip that dose entirely and wait until their next regularly scheduled dose to resume treatment. Doubling up on doses or taking extra tablets to make up for missed doses should never be done, as this increases the risk of side effects without providing additional therapeutic benefit. Patients who frequently forget doses should consider setting reminders or alarms and should discuss adherence strategies with their healthcare provider to maintain consistent therapeutic levels.
How should I handle the oral disintegrating tablets?
Proper handling of Tirzepatide ODT tablets is essential for maintaining their integrity and ensuring proper administration. Tablets should remain in their original packaging until immediately before use to protect them from moisture and maintain their rapid-dissolving properties. Hands should be completely dry when handling the tablets, as moisture can cause premature dissolution. The tablet should be removed carefully from its packaging and immediately placed on the tongue, where it will dissolve within thirty to sixty seconds without the need for water. Patients should not push the tablet through foil packaging if present, as this could damage the tablet. The tablet should not be split, crushed, or chewed, as this could affect its dissolution characteristics and potentially its absorption. If a tablet is accidentally dropped or appears damaged, it should be discarded and a new tablet used.
Administration Instructions
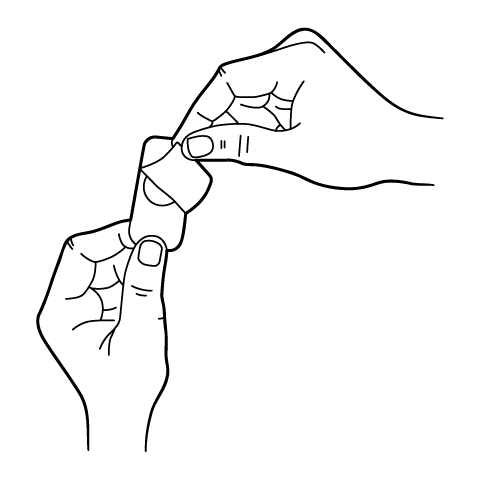
ODT and Troches Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Ondansetron ODT
Ondansetron ODT Sermorelin Acetate Injection
Sermorelin Acetate Injection Hydrochlorothiazide Tablets
Hydrochlorothiazide Tablets Testosterone Cypionate / Testosterone Propionate Injection
Testosterone Cypionate / Testosterone Propionate Injection Lisinopril Tablets
Lisinopril Tablets Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection Lipo Injection
Lipo Injection Lipo Burn Capsules
Lipo Burn Capsules Ondansetron Tablets
Ondansetron Tablets 7-Keto DHEA Capsules
7-Keto DHEA Capsules