Product Overview
Hair Restore MFT Scalp Solution is a pharmacist-compounded topical preparation that combines three active agents-minoxidil 5 %, finasteride 0.3 %, and tretinoin 0.01 %-in a hydro-alcoholic vehicle supplied in a 60 mL bottle intended for direct scalp application. The formulation is dispensed only pursuant to a patient-specific prescription under section 503A, meaning each batch is prepared for an individual and conforms to United States Pharmacopeia standards for potency, sterility, and beyond-use dating, yet it is not evaluated by the United States Food and Drug Administration for safety or efficacy. Clinicians often reserve compounded multi-ingredient solutions for patients whose androgenetic alopecia or other non-scarring alopecias have proven refractory to monotherapy, or for those who wish to streamline separate topical regimens into a single vehicle. Interest in fixed-dose combinations has been amplified by randomized data showing that dual or triple actives can improve mean hair shaft diameter, phototrichogram counts, and patient-reported satisfaction without proportionally increasing adverse events, supporting the rationale for an integrated product such as Hair Restore MFT.[1]
Alopecia affects roughly half of men and a substantial proportion of women by mid-life and is increasingly recognized as a condition that may coexist with metabolic syndrome, psychological distress, and diminished quality of life. Epidemiologic surveys reveal higher rates of insulin resistance, dyslipidemia, and polycystic ovary syndrome in individuals with early-onset androgenetic alopecia, underscoring the need for comprehensive management that extends beyond cosmetic concerns. Because scalp miniaturization evolves gradually and relentlessly, early pharmacologic intervention is critical; however, patient adherence is notoriously poor when separate dropper bottles or foams are required twice daily. A compounded one-step preparation can therefore improve persistence by simplifying the routine and by allowing the prescriber to titrate individual concentrations in response to efficacy or intolerance. These practical considerations, together with emerging stability data for modern solvents such as polysorbate-based carriers, position Hair Restore MFT as a pragmatic option within evidence-informed hair restoration algorithms.[2]
The suggested initial regimen is one full dropper (roughly 1 mL) applied to the affected scalp areas twice daily, ideally twelve hours apart, massaged gently with fingertip pressure for thirty seconds to enhance follicular penetration. Clinical trials with similar strengths achieved optimal density gains at daily doses not exceeding 2 mL; higher volumes saturate the scalp without conferring extra benefit and may elevate systemic exposure. Patients should apply the solution to dry hair and scalp, avoid washing for four hours, and shampoo once daily to remove residual excipients. Visible regrowth typically commences at twelve to sixteen weeks, with maximal effect by twelve months; treatment must continue indefinitely to sustain results. If excessive irritation develops, clinicians may reduce tretinoin concentration, shift to once-daily evening application, or institute pulse dosing every other night while maintaining the morning minoxidil-finasteride component.[9]
Minoxidil, originally developed as an oral antihypertensive, is a pro-drug whose sulfated metabolite opens ATP-sensitive potassium channels in dermal papilla cells, prolonging anagen, enlarging follicular size, and shortening telogen latency. Topical delivery achieves high follicular concentrations while minimizing systemic vasodilatory exposure; optical coherence tomography studies indicate earlier entry of telogen follicles into anagen and a visible increase in bulb diameter within weeks of initiation.[3]
Finasteride, a selective competitive inhibitor of type II 5-α-reductase, lowers dihydrotestosterone in scalp tissue and thereby attenuates androgen-mediated miniaturization. While oral finasteride reduces serum dihydrotestosterone by about 70 %, topical micro-dosed solutions attain nanomolar follicular levels with negligible systemic absorption, limiting sexual or neuropsychiatric sequelae. Synergy with minoxidil is multifactorial: minoxidil accelerates follicular cycling, whereas finasteride preserves terminal caliber; together they offer complementary stimulation and maintenance. Tretinoin adds a further dimension by increasing epidermal turnover, disrupting the stratum corneum, and up-regulating follicular sulfotransferase that converts minoxidil to its active metabolite, a mechanism confirmed in pharmacodynamic crossover trials where topical tretinoin rescued prior minoxidil non-responders.[4]
Hair Restore MFT is contraindicated in anyone with known hypersensitivity to minoxidil, finasteride, tretinoin, propylene glycol, ethanol, or other formulation excipients; in patients with active scalp dermatoses such as erosive lichen planus, severe seborrheic dermatitis, or chronic folliculitis until those conditions are quiescent; and in individuals with unstable cardiovascular disease where inadvertent systemic absorption of minoxidil could exacerbate fluid retention or tachycardia. Use on abraded or sun-burned skin heightens absorption risk and should be avoided. Because finasteride is teratogenic to male fetuses, contact with the product is absolutely contraindicated in women who are pregnant or may become pregnant, and gloves are recommended for caregivers applying the solution to another person. Patients receiving systemic retinoids must not add topical tretinoin owing to cumulative irritation, and anyone with uncontrolled hypertension or orthostatic hypotension should first achieve cardiovascular stability before embarking on therapy.[5]
Although topical delivery limits systemic exposure, clinicians should account for cutaneous and potential systemic interactions. Finasteride is metabolized primarily by cytochrome P450 3A4; therefore, potent cutaneous CYP3A4 inhibitors (e.g., ketoconazole shampoo left in place for prolonged periods) could conceivably elevate local finasteride levels, while strong inducers such as chronic topical corticosteroids may diminish effect. If systemic azoles, macrolides, or anticonvulsants are prescribed concurrently, trace systemic finasteride exposure could be amplified or reduced, albeit clinical significance remains theoretical. Minoxidil shares no major cytochrome pathways, yet profound vasodilation can theoretically intensify hypotension if patients later require systemic antihypertensives or phosphodiesterase-5 inhibitors. Occlusive dressings, microneedling, or low-level laser helmets used immediately before application may increase percutaneous absorption of all actives and thereby magnify adverse reactions. Given the retinoid component, concomitant use of other keratolytics, chemical peels, or ultraviolet phototherapy raises cumulative phototoxicity, warranting extended sun protection and staggered scheduling.[6]
In real-world surveillance, the most common adverse events are dose-dependent scalp pruritus, erythema, and transient telogen effluvium during the first two months of treatment-a reflection of follicles synchronously entering anagen. Contact dermatitis occurs in up to 6 % of users and is often attributable to propylene glycol rather than minoxidil itself; switching to a low-glycol or foam vehicle can mitigate symptoms. Diffuse facial hypertrichosis has been reported in women, usually when application extends beyond the frontal hairline. Topical finasteride rarely leads to measurable serum drug levels, yet isolated narratives describe decreased libido or mood changes resembling post-finasteride syndrome; these resolve after discontinuation but underscore the need for informed consent. Tretinoin contributes predictable desquamation, burning, and photosensitization that respond to alternating-day use or post-application emollients. Severe reactions-angioedema, wheezing, hypertensive crisis-are exceedingly rare and constitute grounds for immediate cessation and medical evaluation.[7]
Animal and human case data designate oral finasteride as pregnancy category X owing to the risk of feminization of male fetuses, and topical exposure-while lower-remains contraindicated for the same reason.
Minoxidil is category C; teratogenicity has not been demonstrated, but neonatal hypertrichosis and cardiovascular anomalies have been described after maternal ingestion.
Topical tretinoin belongs to category C and, although systemic absorption is minimal, retinoid embryopathy cannot be excluded.
For these reasons, Hair Restore MFT must not be prescribed to pregnant or nursing individuals, and secondary exposure should be prevented by thorough handwashing, storage out of reach of children, and avoidance of pillow or towel contamination.
Pregnancy testing should precede therapy in women of childbearing potential, and effective contraception maintained throughout treatment and for at least one full hair cycle (approximately six months) after discontinuation.[8]
The solution should be stored tightly capped at controlled room temperature-20 ° to 25 °C (68 ° to 77 °F)-and shielded from direct sunlight to preserve minoxidil stability and prevent finasteride photodegradation. Do not freeze; crystallization may occur below 15 °C, reducing potency and altering dropper calibration. Patients should keep the bottle in a dry cabinet rather than a humid bathroom, wipe the dropper after each use to limit microbial ingress, and discard any remaining product beyond the pharmacist-assigned beyond-use date. Color changes toward a yellow-brown hue or the appearance of sediment signal oxidation and warrant replacement.[10]
- Iamsumang, W., Suchonwanit, P., & Rojhirunsakool, S. (2018). Efficacy and safety of topical combination of 3 % minoxidil and 0.25 % finasteride versus 3 % minoxidil solution in female-pattern hair loss: A randomized, double-blind, controlled study. Journal of the American Academy of Dermatology, 79(4), 832-838. https://doi.org/10.1016/j.jaad.2018.02.044
- Chen, S., Xie, X., Zhang, G., & Zhang, Y. (2022). Comorbidities in androgenetic alopecia: A comprehensive review. Dermatology and Therapy, 12(10), 2233-2247. https://doi.org/10.1007/s13555-022-00799-7
- Messenger, A. G., & Rundegren, J. (2004). Minoxidil: Mechanisms of action on hair growth. British Journal of Dermatology, 150(2), 186-194. https://doi.org/10.1111/j.1365-2133.2003.05299.x
- Lubis, F. F., Legiawati, L., Saulina, M., & Saldi, S. R. F. (2025). Randomized controlled trial on the efficacy and safety of combination therapy of topical 0.1 % finasteride and 5 % minoxidil in male androgenetic alopecia. Archives of Dermatological Research, 317, 691. https://doi.org/10.1007/s00403-025-04216-9
- Drugs.com. (2023, August 9). Minoxidil (Loniten) use during pregnancy. https://www.drugs.com/pregnancy/minoxidil.html
- Indiana University School of Medicine. (2025). Cytochrome P450 drug interaction table. https://drug-interactions.medicine.iu.edu/MainTable.aspx
- GoodRx Health. (2025, April 3). 5 topical minoxidil side effects you should know about. https://www.goodrx.com/minoxidil/topical-minoxidil-side-effects
- Drugs.com. (2025). Finasteride use during pregnancy. https://www.drugs.com/pregnancy/finasteride.html
- Drugs.com. (2025, May 15). Minoxidil topical dosage guide. https://www.drugs.com/dosage/minoxidil-topical.html
- PonteVitaRx. (2025, June 21). 12-month minoxidil supply: Benefits and storage tips. https://pontevitarx.com/12-month-minoxidil-supply-benefits-storage/
- GoodRx Health. (2025, March 30). Minoxidil dosages: Formulations, strengths, and adjustments. https://www.goodrx.com/minoxidil-non-prescription/dosage
- Cohen, S., et al. (2001). Allergic contact dermatitis to topical minoxidil solution: Etiology and cross-reactivity. Journal of the American Academy of Dermatology, 45(4), 613-618. https://doi.org/10.1067/mjd.2001.117893
- DermNet. (2025). Contact allergy to propylene glycol. https://dermnetnz.org/topics/contact-allergy-to-propylene-glycol
- González, J., et al. (1992). Influence of tretinoin on the percutaneous absorption of minoxidil from an aqueous topical solution. Journal of Pharmaceutical Sciences, 81(6), 481-485. https://doi.org/10.1002/jps.2600810606
- U.S. Food and Drug Administration. (2025, May 10). FDA alerts health care providers to potential risks associated with compounded topical finasteride products. https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-consumers-potential-risks-associated-compounded
- Epocrates. (2025, May 12). FDA warns of risks with compounded topical finasteride. https://www.epocrates.com/online/article/fda-warns-of-risks-with-compounded-topical-finasteride
- Mayo Clinic. (2025). Minoxidil (topical route). https://www.mayoclinic.org/drugs-supplements/minoxidil-topical-route/
- Hairguard. (2024). Minoxidil once or twice a day? https://www.hairguard.com/minoxidil-once-a-day/
- DrHair. (2025, February 20). How often should I apply minoxidil 5 %? https://drhair.co.uk/blog/how-often-should-i-apply-minoxidil/
- U.S. Food and Drug Administration. (2012). Propecia (finasteride) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf
When should I expect visible improvement in hair density and coverage?
Consistent twice-daily use is usually required for three to four months before new terminal hairs become apparent, with progressive gains up to one year.[11]
Why is tretinoin included if I already tolerate minoxidil and finasteride?
Tretinoin can enhance follicular uptake of minoxidil by loosening the stratum corneum and up-regulating activating enzymes, improving response in prior non-responders.[12]
My scalp itches after application-is that normal?
Mild pruritus is common and often relates to propylene glycol; substitution with alternative vehicles or adding a silicone-based barrier serum usually alleviates discomfort.[13]
Does tretinoin make the scalp more sensitive to sunlight?
Yes, tretinoin accelerates epidermal turnover and reduces the minimal erythema dose, so broad-spectrum sunscreen or a hat is advised during daylight exposure.[14]
Can topical finasteride still cause systemic side effects?
Systemic absorption is low, but the FDA has documented cases of sexual and neuropsychiatric events; patients should report any persistent symptoms promptly.[15]
Is this compounded product FDA-approved?
No; compounded topical finasteride and multi-ingredient solutions lack FDA approval and are dispensed under 503A pharmacy regulations, so safety data are limited.[16]
How should I apply the solution to avoid run-off and facial hair growth?
Keep the dropper tip close to the scalp, part the hair in rows, and pat lightly; wash hands thoroughly afterward to prevent unwanted exposure.[17]
Is once-daily dosing ever sufficient?
Some data suggest once-daily application may maintain benefit after the first year, but twice-daily use provides superior density during the initial treatment period.[18]
What happens if I miss a dose?
Resume the next scheduled application without doubling; sporadic lapses are unlikely to reverse gains if overall adherence remains high.[19]
Are there special precautions for partners who may become pregnant?
Partners who are or may become pregnant should avoid contact with treated scalp areas or linens for at least four hours after application, and caregivers should use gloves.[20]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
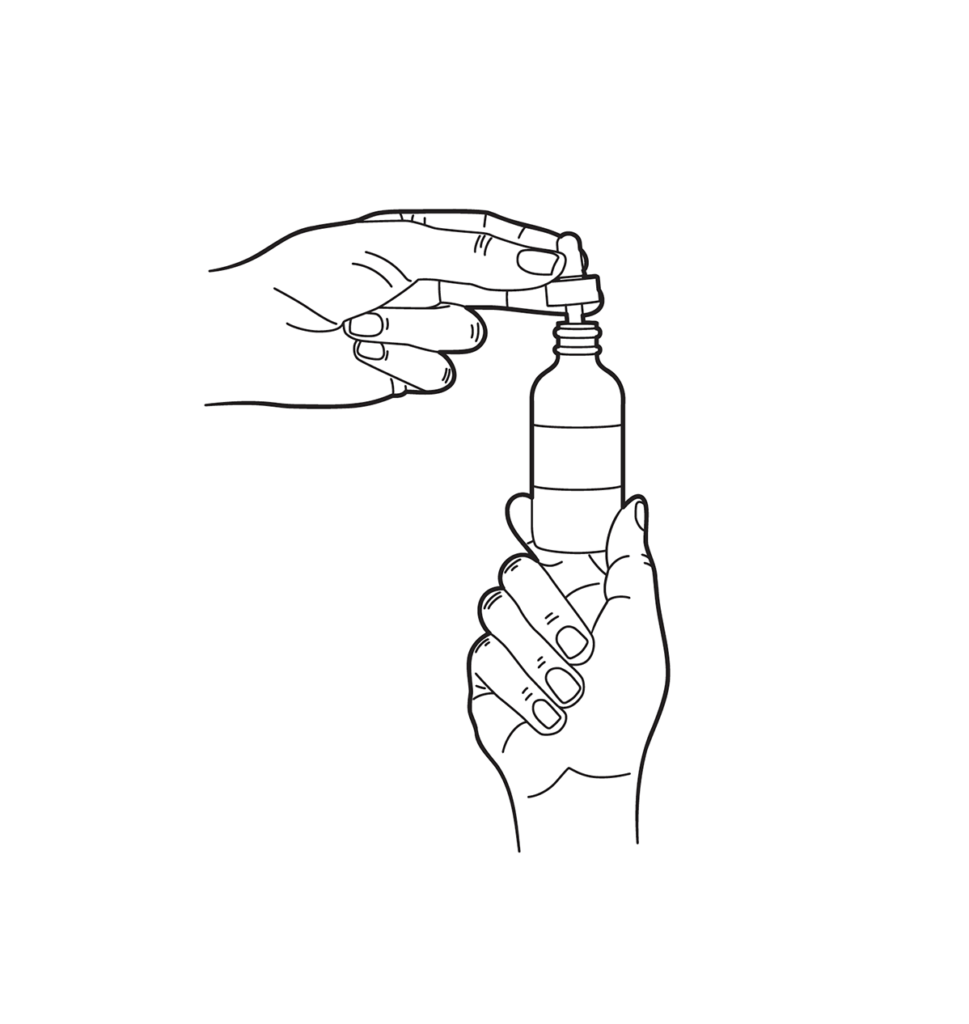
Dropper Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 GHK-Cu Scalp Solution
GHK-Cu Scalp Solution Hair Restore D Scalp Solution
Hair Restore D Scalp Solution Hair Restore MDT Scalp Solution
Hair Restore MDT Scalp Solution Minoxidil Capsules
Minoxidil Capsules Dutasteride Capsules
Dutasteride Capsules Finasteride Tablets
Finasteride Tablets Biotin / Minoxidil / Spironolactone Capsules
Biotin / Minoxidil / Spironolactone Capsules Biotin / Minoxidil Capsules
Biotin / Minoxidil Capsules Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection Hair Restore Ultra Scalp Solution
Hair Restore Ultra Scalp Solution