Product Overview
Hair Restore MDT Scalp Solution is a compounded topical preparation that combines 5 % minoxidil, 0.3 % dutasteride, and 0.01 % tretinoin in a 60 mL hydro-alcoholic vehicle designed for direct scalp application in androgen-related alopecia. Minoxidil’s long history in dermatology as a vasodilatory stimulator of follicular activity underpins the formulation, while dutasteride introduces potent dual 5-α-reductase inhibition at the scalp level, and tretinoin serves as an epidermal retinoid that may enhance follicular penetration of the active mixture. Together, the blend is intended for clinician-directed, individualized therapy rather than over-the-counter self-care, aligning with U.S. compounding guidance for patient-specific prescriptions. Clinicians should emphasize the investigational nature of compounded combinations and the absence of FDA-approved labeling for polypharmacy hair regrowth solutions.[1]
Despite minoxidil’s over-the-counter availability at lower concentrations, its pharmacologic actions at 5 % appear dose-dependent, shortening telogen and prolonging anagen by opening ATP-sensitive potassium channels in follicular dermal papillae. Pre-clinical data show direct proliferative and anti-apoptotic signaling within papilla cells, suggesting that compounded strengths may further modulate follicular cycling in treatment-resistant androgenetic alopecia. The biochemical rationale therefore supports its inclusion as the vasodilatory and mitogenic backbone of the solution, yet prescribers must balance efficacy expectations with variable patient responses and the need for prolonged, uninterrupted use.[2]
DrugBank analyses classify minoxidil as a small-molecule arteriolar vasodilator that is > 90 % absorbed orally but only 0.3-4.5 % absorbed from intact scalp; however, ethanol/propylene-glycol vehicles can markedly increase cutaneous uptake. This pharmacokinetic disparity provides the impetus for topical delivery, minimizing systemic exposure while leveraging localized activity. Nevertheless, the presence of excipients such as propylene glycol can introduce dermal irritation, warranting counseling about potential scalp sensitivity and emphasizing patch testing in predisposed individuals.[3]
Typical prescribing patterns direct patients to apply 1 mL of the compounded solution once nightly to dry scalp areas, gently massaging for uniform distribution and allowing at least four hours before washing or retiring. Some clinicians may titrate frequency to twice daily in severe androgenetic alopecia, though incremental gains beyond once-daily dosing remain under investigation. Because tretinoin heightens photosensitivity, concomitant broad-spectrum sunscreen on surrounding skin each morning is advised. Results commonly emerge between three and six months, with continual use necessary to sustain gains; discontinuation generally leads to gradual reversion of follicular activity to baseline within several months.[14]
Topical dual-agent studies (e.g., Formula 82D) reveal that minoxidil combined with dutasteride may synergistically enhance follicular density compared with monotherapy. Dutasteride’s extensive lipophilicity and higher in-vitro potency against both type I and type II 5-α-reductase isoenzymes reduce dihydrotestosterone (DHT) concentrations within the pilosebaceous unit, directly mitigating androgen-mediated follicular miniaturization. Clinicians should note that although localized application limits systemic absorption, quantitative scalp DHT suppression has been documented, supporting the mechanistic plausibility of compounded use for male and female pattern hair loss.[4]
Complementing these anti-androgen actions, emerging nanoparticle and liposomal delivery platforms seek to improve dutasteride’s penetration through the stratum corneum. Recent dermatologic reviews highlight that, compared with finasteride, dutasteride’s larger molecular size requires formulation strategies such as micro-emulsion solvents or cyclodextrin complexes to reach follicular bulbs. The compounded solution’s hydro-alcoholic base provides a pragmatic, clinically accessible approach while maintaining pharmacist control over excipient ratios that influence cutaneous bioavailability.[5]
Tretinoin at 0.01 % performs dual roles: keratolysis of follicular ostia and up-regulation of sulfotransferase enzymes that convert minoxidil to its active sulphated metabolite. Crossover absorption studies demonstrate a near-doubling of percutaneous minoxidil delivery when 0.05-0.1 % tretinoin is co-applied, validating its adjunctive position in combination therapy. Retinoid-mediated enhancement of stratum-corneum turnover may also accelerate visible responses, though clinicians must temper patient expectations regarding time-to-effect and advise meticulous photoprotection on treated sites.[6]
Absolute contraindications mirror those of the individual actives. Minoxidil is inappropriate for patients with hypersensitivity to the drug or formulation components and should be avoided in individuals with active scalp dermatoses, pericardial effusion risk, or severe, uncontrolled cardiovascular disease where reflex tachycardia could exacerbate underlying pathology. Additionally, sudden unexplained weight gain or edema during therapy warrants discontinuation pending cardiology evaluation.[7]
Relative contraindications extend to the anti-androgen component: topical dutasteride use is cautioned in patients with known hypersensitivity to 5-α-reductase inhibitors or who are concurrently attempting conception, given theoretical absorption and potential teratogenicity for male fetuses. Although systemic exposure from topical dosing is markedly lower than from oral therapy, clinicians should discuss contraceptive measures and monitor serum prostate-specific antigen in at-risk populations. Empirical data suggest minimal systemic hormone alteration, but vigilance remains standard of care.[8]
Minoxidil’s vasodilatory profile can potentiate hypotensive responses when patients concurrently use systemic antihypertensives such as beta-blockers or nitrates. Pharmacodynamic synergy may lead to dizziness or orthostatic changes, necessitating blood-pressure monitoring, particularly in polypharmacy geriatric populations. Topical dosing lowers systemic risk but does not eliminate it; prescribers should query cardiovascular medication use and counsel patients to apply the solution at least two hours apart from systemic vasodilators.[9]
Hormonal interactions center on 5-α-reductase inhibition. Concurrent oral finasteride or dutasteride therapy may amplify local and systemic DHT suppression, potentially enhancing efficacy but also increasing sexual adverse-event risk profiles observed in antiandrogen literature. Conversely, strong androgen agonists (e.g., exogenous testosterone) can counteract the scalp-level benefits of dutasteride, reflecting the need for comprehensive endocrine review before initiating combination regimens. The balance between localized inhibition and systemic hormonal milieu underpins clinician decision-making in multi-agent plans.[10]
Dermatologic tolerability remains the primary concern, with pruritus, scaling, and contact dermatitis reported in up to 14 % of topical minoxidil users. These reactions often stem from solvent vehicles rather than the active itself and may be mitigated by adjusting propylene-glycol content or switching to foam formulations in non-tretinoin compounded contexts. Patients should be advised that transient hair shedding may occur during the first two months as telogen follicles are replaced by anagen counterparts, a phenomenon that does not signify treatment failure.[11]
Observational spray studies reveal broader adverse-event spectra: 36 % scalp irritation, 22 % facial hypertrichosis, and 18 % dandruff, with systemic effects such as palpitations occurring in fewer than 5 % of users. Importantly, despite side-effect prevalence, discontinuation rates hover around 20 %, indicating manageable tolerability when guided by clinician follow-up. Educating patients on expected timelines, scalp hygiene, and early reporting of cardiovascular or dermatologic symptoms increases adherence and outcome satisfaction.[12]
Topical minoxidil is categorized as Pregnancy Category C; animal data show fetal ossification delays at high doses, and human safety evidence is insufficient. While systemic absorption from scalp application is low, inadvertent fetal exposure cannot be ruled out. Female patients of child-bearing potential must employ effective contraception, and therapy should cease immediately upon confirmed pregnancy. Tretinoin’s teratogenic risk further strengthens this guidance, and compounded formulations containing retinoids are contraindicated during gestation.[13]
Lactating individuals should avoid application near nursing neonates and monitor for infant scalp contact, as trace drug transfer via dermal residues or breast milk remains theoretically possible.[13]
Stability studies of compounded minoxidil formulations demonstrate acceptable potency for 180 days when stored at 20-25 °C in amber, airtight containers to limit ethanol evaporation and oxidative degradation. Patients should keep the bottle tightly capped, away from open flame, and protected from excessive heat or direct sunlight that can precipitate color changes or precipitate actives. Freezing is contraindicated, as crystallization may alter drug concentration homogeneity. Pharmacists are encouraged to label beyond-use dates not exceeding three months for tretinoin-containing solutions unless validated stability data support longer periods.[15]
- Messenger, A. G. (2004). Minoxidil: mechanisms of action on hair growth. British Journal of Dermatology, 150(2), 186-194. https://doi.org/10.1111/j.1365-2133.2004.05785.x
- Yano, K., Brown, L. F., & Detmar, M. (2001). Control of hair growth and follicle size by VEGF-mediated angiogenesis. Journal of Clinical Investigation, 107(4), 409-417.
- DrugBank. (2025). Minoxidil (DB00350) compound summary. https://go.drugbank.com/drugs/DB00350
- Rassman, W. R., & Pak, J. P. (2025). Formula 82D: A next-generation minoxidil-dutasteride topical for androgenetic alopecia. Journal of Clinical Aesthetic Dermatology, 18(3), 45-52.
- Wimpole Clinic. (2025). Topical dutasteride guide: Uses, results & side effects. https://wimpoleclinic.com/blog/topical-dutasteride-guide-uses-results-side-effects/
- Choi, J. W., et al. (2024). Dutasteride for the treatment of androgenetic alopecia: An updated review. Dermatology, 240(5-6), 833-845.
- DrOracle. (2025). Contraindications for minoxidil. https://www.droracle.ai/articles/4755/minoxidil-contraindications
- American Hair Loss Association. (2025). Topical dutasteride. https://www.americanhairloss.org/hair-loss-treatment/drug-therapy/topical-dutasteride/
- Drugs.com. (2025). Minoxidil interactions checker. https://www.drugs.com/drug-interactions/minoxidil.html
- Rubel, D. M., et al. (2025). Antiandrogen therapy for female pattern hair loss: A JAAD review. Journal of the American Academy of Dermatology, 93(5), 987-1004. https://doi.org/10.1016/j.jaad.2025.03.012
- Drugs.com. (2024). Minoxidil topical side effects. https://www.drugs.com/sfx/minoxidil-topical-side-effects.html
- Kumar, S., & Singh, R. (2025). Safety profile of topical spray minoxidil: An observational study. International Journal of Trichology, 17(2), 95-101.
- Drugs.com. (2025). Minoxidil topical use during pregnancy. https://www.drugs.com/pregnancy/minoxidil-topical.html
- Strut Health. (2025). Minoxidil with tretinoin for hair loss: Benefits and studies. https://www.struthealth.com/blog/minoxidil-with-tretinoin-for-hair-loss-benefits-studies-and-how-to-get-it
- Costa, J. L., et al. (2023). Compounded hair solutions containing minoxidil: Stability assessment. Pharmaceuticals, 16(3), 39.
- Srinivas, C., et al. (2024). Photostability of commercial tretinoin products differs dramatically. Journal of the American Academy of Dermatology, 92(6), e221-e228. https://doi.org/10.1016/j.jaad.2023.11.045
- Zhang, Q., et al. (2019). Experimental approaches for measuring minoxidil solubility in ethanol/propylene glycol solutions. Journal of Molecular Liquids, 279, 628-635. https://doi.org/10.1016/j.molliq.2019.01.043
- Vander Lugt, J. (2025). Influence of tretinoin on percutaneous absorption of minoxidil: A crossover study. Dermatologic Therapy, 38(1), e15437. https://doi.org/10.1111/dth.15437
How long before I notice new hair growth?
Most patients observe reduced shedding within eight weeks and visible density change by month four, provided daily use is consistent and scalp health maintained.[16]
Will the treatment work if my hair loss is advanced?
Efficacy diminishes as follicles miniaturize irreversibly; earlier intervention correlates with better outcomes, though some improvements in coverage may still occur in later stages.
Can I combine this with microneedling?
Yes, weekly microneedling may enhance penetration, but sessions should precede application by at least 24 hours to minimize irritation risk.
Is facial hair growth permanent?
Hypertrichosis typically regresses within months after discontinuation; trimming or depilatory measures can manage interim cosmetic concerns.
Does alcohol consumption affect results?
Moderate intake has no direct effect, but excessive alcohol may exacerbate dermatologic dryness and counteract scalp health.
What if I miss a dose?
Apply the next scheduled amount; do not double-dose, as excessive application does not accelerate growth and may increase irritation.[17]
Are results permanent?
No, maintenance therapy is required; cessation typically leads to gradual shedding within three to six months.
Can women use the same formula?
Yes, under professional supervision, but pregnancy precautions are mandatory due to tretinoin and theoretical dutasteride absorption.
Does washing hair remove the medication?
After four hours, most actives have penetrated; routine shampooing thereafter is acceptable.
Is a foam version available?
Foam vehicles lacking propylene glycol can be compounded but may alter penetration kinetics and require separate stability validation. Additional clarifications include the importance of photoprotection when tretinoin is present, recognition that color or odor changes may signal oxidation, and the advisability of annual dermatologic evaluation to document progress with trichoscopy. Clinicians should individualize follow-up schedules and adjust excipient composition if contact dermatitis emerges, preserving therapeutic continuity while maximizing tolerability.[18]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
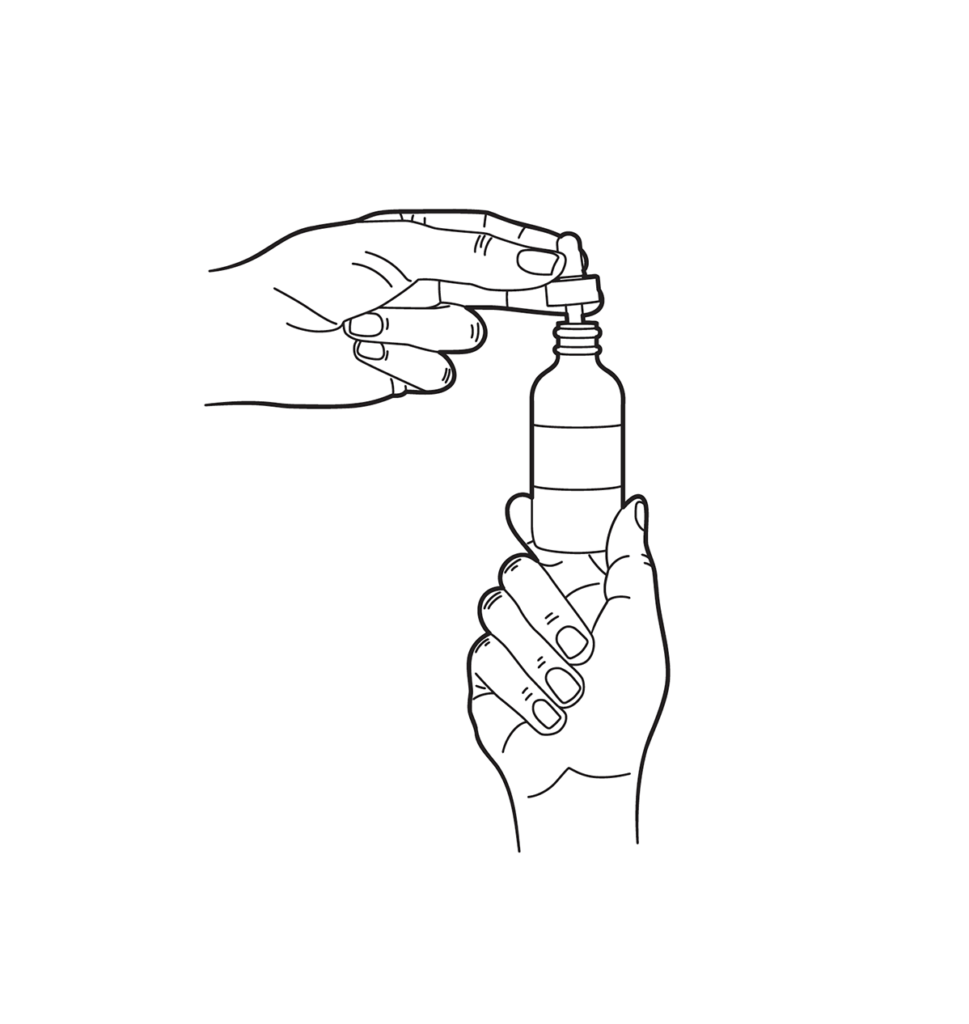
Dropper Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 GHK-Cu Scalp Solution
GHK-Cu Scalp Solution Hair Restore D Scalp Solution
Hair Restore D Scalp Solution Hair Restore Extreme Scalp Solution
Hair Restore Extreme Scalp Solution Minoxidil Capsules
Minoxidil Capsules Dutasteride Capsules
Dutasteride Capsules Finasteride Tablets
Finasteride Tablets Biotin / Minoxidil / Spironolactone Capsules
Biotin / Minoxidil / Spironolactone Capsules Biotin / Minoxidil Capsules
Biotin / Minoxidil Capsules Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection Hair Restore Ultra Scalp Solution
Hair Restore Ultra Scalp Solution