Cholecalciferol (D3) Injection
Product Overview
Cholecalciferol (Vitamin D₃) Injection 100,000 IU/mL is a sterile, oil-based compounded solution prepared in grapeseed oil for deep intramuscular use when patients require rapid or dependable vitamin D repletion because oral preparations are ineffective, poorly absorbed, or impractical. Each 5 mL vial contains a total of 500,000 IU of vitamin D₃ suspended in an antioxidant-rich plant oil that is well tolerated by muscle tissue. Pharmacies operating under section 503A compound the medication for patient-specific prescriptions, whereas a registered 503B outsourcing facility can prepare office-use stock for clinics that administer injections on site, ensuring current Good Manufacturing Practice and USP <797> compliance.[2]
High-dose intramuscular administration creates a tissue depot that releases vitamin D slowly over weeks, potentially enabling a single injection to lift serum 25-hydroxyvitamin D levels into the sufficient range in severe deficiency and to potentially support bone mineralisation, neuromuscular function, and secondary hyperparathyroidism reversal while bypassing the gastrointestinal tract.[3]
A common repletion strategy in profoundly deficient adults involves 100,000 IU intramuscularly every four weeks for two or three doses or a one-off 300,000 IU injection followed by oral maintenance; experienced endocrinologists may individualise based on baseline 25-hydroxyvitamin D, body mass, malabsorption severity, and season.[5]
Serum 25-hydroxyvitamin D, calcium, phosphate, and parathyroid hormone are re-examined at least eight weeks after each injection, and maintenance may shift to oral 1,000-2,000 IU daily once target levels (generally 30-50 ng mL ⁻¹) are achieved, thereby reducing the risk of cumulative overdose while safeguarding skeletal health.[17]
If a scheduled injection is missed, patients should arrange administration as soon as practicable and resume the original calendar rather than double the dose; they should avoid unsupervised over-the-counter vitamin D products because combined intake can unknowingly exceed tolerable upper limits.[21]
After the solution is deposited into muscle, cholecalciferol diffuses into the circulation bound to vitamin-D-binding protein and undergoes 25-hydroxylation in hepatocytes, yielding 25-hydroxyvitamin D, the principal circulating biomarker of status.[4]
The second activation step takes place predominantly in the kidneys where 1α-hydroxylase converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D (calcitriol), an active secosteroid hormone that binds nuclear receptors in enterocytes, osteoblasts, renal tubules, parathyroid cells, and numerous immune and endocrine cells to modulate hundreds of genes involved in calcium-phosphate homeostasis, skeletal remodelling, and immunoregulation.[5]
Administering 100,000 IU intramuscularly establishes a high-capacity depot that may correct deficiency more predictably than oral dosing in malabsorption syndromes and maintains adequate circulating vitamin D over two to three months, an approach often referred to as ‘Stoss therapy’ in endocrinology.[6]
Absolute contraindications include hypercalcaemia, hypervitaminosis D, idiopathic infantile hypercalcaemia, or documented hypersensitivity to vitamin D₃ or components of the oil vehicle because additional vitamin D would worsen calcium overload or provoke severe allergic reactions.[7]
Clinicians should exercise extreme caution in advanced chronic kidney disease or sarcoidosis, tuberculosis, and other granulomatous disorders where extrarenal 1α-hydroxylase activity can generate excess calcitriol and precipitate hypercalcaemia even at modest serum 25-hydroxyvitamin D concentrations.[8]
Patients receiving cardiac glycosides, particularly digoxin, may develop life-threatening arrhythmias if hypercalcaemia occurs; pregnancy and lactation warrant specialist oversight since megadose therapy is generally reserved for severe, documented deficiency that cannot be corrected by standard oral supplementation.[9]
Concomitant administration of large calcium supplements, thiazide diuretics, or aluminium-containing phosphate binders can potentiate vitamin D-mediated increases in serum calcium or phosphate, demanding periodic laboratory monitoring and dosage adjustments to avert nephrolithiasis or ectopic calcification.[10]
Enzyme-inducing anticonvulsants such as phenytoin, carbamazepine, and phenobarbital, as well as rifampicin, accelerate hepatic catabolism of vitamin D, shortening its half-life and potentially necessitating more frequent or higher intramuscular doses to sustain target 25-hydroxyvitamin D levels.[11]
Bile-acid sequestrants and orlistat impair intestinal absorption of fat-soluble vitamins, explaining why some patients switch to parenteral therapy, whereas magnesium-containing antacids and digoxin can synergise with vitamin D to provoke hypermagnesaemia or digitalis toxicity if calcium rises unchecked.[12]
Most recipients experience no systemic symptoms; however, transient injection-site pain, mild headache, gastrointestinal upset, or fatigue can occur, and patients should be counselled to report persistent anorexia, constipation, or polyuria that may herald rising serum calcium.[7]
Vitamin D toxicity presents chiefly as sustained hypercalcaemia with nausea, vomiting, muscle weakness, neurocognitive changes, or arrhythmias, progressing to nephrocalcinosis if untreated; toxicity remains rare when clinicians restrict injections to clinically indicated doses and monitor biochemical profiles.[12]
Very high cumulative exposure over months may encourage calcium deposition in soft tissues such as the renal parenchyma or coronary arteries, an outcome implicated in observational studies linking supra-physiological 25-hydroxyvitamin D levels with vascular calcification and heightened fracture risk in frail elders.[13]
Physiological vitamin D sufficiency is vital for placental function, foetal bone accretion, and maternal skeletal health, yet professional bodies caution against parenteral megadose therapy during uncomplicated gestation, preferring daily or weekly oral regimens that allow tighter titration and minimise abrupt calcium flux.[14]
Small clinical trials indicate that maternal supplementation of 4,000 IU day ⁻¹ or staggered 50,000 IU oral doses can safely maintain 25-hydroxyvitamin D above 30 ng mL ⁻¹ without inducing hypercalcaemia, whereas single injections of 300,000 IU are reserved for severe malabsorption emergencies when benefits outweigh theoretical teratogenic risk.[15]
During lactation, parenteral vitamin D markedly raises breast-milk concentrations and can support infant status, yet guidelines still advocate infant drops of 400 IU day ⁻¹ and recommend maternal monitoring to ensure neither mother nor child approaches toxic thresholds.[16]
Vials should be stored upright at 20-25 °C in the original light-resistant container, protected from moisture and heat sources, and gently rolled between the palms to redissolve any oil stratification before withdrawal without vigorous shaking that can introduce air bubbles.[18]
- MedlinePlus. (2024). Cholecalciferol (Vitamin D3). National Library of Medicine. Retrieved June 8, 2025, from https://medlineplus.gov/druginfo/meds/a620058.html
- National Institutes of Health, Office of Dietary Supplements. (2024). Vitamin D fact sheet for health professionals. Retrieved June 8, 2025, from https://ods.od.nih.gov/factsheets/VitaminD-Consumer/
- Institute of Medicine. (2011). Dietary reference intakes for calcium and vitamin D. National Academies Press.
- Holick, M. F. (2007). Vitamin D deficiency. *The New England Journal of Medicine*, 357(3), 266-281. https://doi.org/10.1056/NEJMra070553
- Diamond, T. H., Ho, K. W., Rohl, P. G., & Meerkin, M. (2005). Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: Efficacy and safety data. *Medical Journal of Australia*, 183(1), 10-12.
- Ilahi, M., Armas, L. A., & Heaney, R. P. (2008). Pharmacokinetics of a single, large dose of cholecalciferol. *The American Journal of Clinical Nutrition*, 87(3), 688-691. https://doi.org/10.1093/ajcn/87.3.688
- Mayo Clinic. (2024). Vitamin D toxicity: Symptoms and causes. Retrieved June 8, 2025, from https://www.mayoclinic.org/diseases-conditions/hypercalcemia/expert-answers/vitamin-d-toxicity/faq-20058108
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. (2017). KDIGO clinical practice guideline update for CKD-MBD. *Kidney International Supplements*, 7(1), 1-59. https://doi.org/10.1016/j.kisu.2017.04.001
- Holick, M. F., et al. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. *Journal of Clinical Endocrinology & Metabolism*, 96(7), 1911-1930. https://doi.org/10.1210/jc.2011-0385
- RxList. (2025). Cholecalciferol drug interactions. Retrieved June 8, 2025, from https://www.rxlist.com/cholecalciferol-drug-interactions.htm
- Patel, A. D., et al. (2016). The effect of antiepileptic drugs on vitamin D metabolism. *Seizure*, 35, 33-37. https://doi.org/10.1016/j.seizure.2015.11.006
- Barton, A., & Wilkins, T. (2012). Vitamin D deficiency and toxicity. *American Family Physician*, 85(11), 1115-1120.
- Cao, Y., et al. (2013). Vitamin D supplementation and fracture risk: A meta-analysis of randomised controlled trials. *PLoS ONE*, 8(12), e79913. https://doi.org/10.1371/journal.pone.0079913
- American College of Obstetricians and Gynecologists. (2021). Clinical guidance on vitamin D during pregnancy and lactation. Retrieved June 8, 2025, from https://www.acog.org/clinical/clinical-guidance
- Wagner, C. L., & Taylor, S. N. (2016). Maternal vitamin D supplementation during pregnancy. *Journal of Pregnancy and Child Health*, 3, 247. https://doi.org/10.4172/2376-127X.1000247
- Pludowski, P., et al. (2018). Vitamin D supplementation guidelines. *Nutrients*, 10(5), 1-20. https://doi.org/10.3390/nu10050573
- UpToDate. (2025). Vitamin D deficiency in adults: Definition, clinical manifestations, and treatment. Wolters Kluwer Health.
- United States Pharmacopeia. (2024). <797> Pharmaceutical Compounding - Sterile Preparations.
- U.S. Food and Drug Administration. (2023). Compounding quality: Current good manufacturing practice for 503B outsourcing facilities. https://www.fda.gov
- U.S. Food and Drug Administration. (2022). Drug disposal: Dispose of unused medicines-properly. https://www.fda.gov/drugs/safe-disposal-medicines
- MedlinePlus. (2024). Calcium in diet. National Library of Medicine. Retrieved June 8, 2025, from https://medlineplus.gov/ency/article/002412.htm
- National Institutes of Health, Office of Dietary Supplements. (2024). Magnesium fact sheet for health professionals. Retrieved June 8, 2025, from https://ods.od.nih.gov/factsheets/Magnesium-Consumer/
- Holick, M. F., & Binkley, N. (2017). Optimal vitamin D status for health: A review of clinical evidence. *Current Opinion in Endocrinology, Diabetes & Obesity*, 24(6), 420-435. https://doi.org/10.1097/MED.0000000000000368
- Harvard T.H. Chan School of Public Health. (2024). Vitamin D and health. Retrieved June 8, 2025, from https://www.hsph.harvard.edu/nutritionsource/vitamin-d/
- Heath, K. M., & Elovic, E. P. (2020). Vitamin D deficiency: Screening and treatment update. *Archives of Physical Medicine and Rehabilitation*, 101(1), 30-35. https://doi.org/10.1016/j.apmr.2019.09.113
How soon will my vitamin D improve?
A single 100,000 IU injection can elevate serum 25-hydroxyvitamin D within four weeks and sustain sufficiency for up to three months, although individual kinetics vary with baseline depletion and body composition.
Does the shot hurt?
Most patients describe brief soreness comparable to a vaccination and can resume normal activity immediately.
Can I self-inject?
Deep gluteal administration is best performed by trained clinicians to minimize nerve or vascular injury.[22]
Will I still need calcium tablets?
Adequate dietary calcium is encouraged, but high-dose supplements should only follow medical advice because vitamin D enhances absorption.
Is sunlight a substitute?
Moderate sunlight contributes, yet reliance on UV exposure risks skin cancer and yields inconsistent vitamin D in darker skin or winter latitudes.
Can medicines interfere?
Anticonvulsants, steroids, and orlistat can lower vitamin D levels or reduce its effect, so dosing may need adjustment.[23]
What signs suggest overdose?
Persistent thirst, frequent urination, nausea, or mental confusion warrant prompt blood tests for calcium.
Does the oil cause weight gain?
The grapeseed carrier adds negligible calories (<5 kcal mL ⁻¹) and is unlikely to affect weight.
Is it vegan-friendly?
Yes; cholecalciferol is synthesized from lanolin but free of animal tissue proteins, and the oil is plant derived.[24]
How long will I stay on injections?
Many patients require only one or two doses, then switch to oral maintenance; those with permanent malabsorption may receive boosters every three to six months, guided by periodic laboratory assessment and clinical response.[25]
Administration Instructions
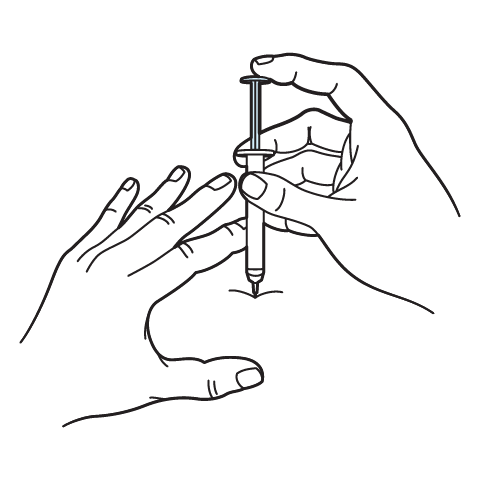
Intramuscular Injection Instructions
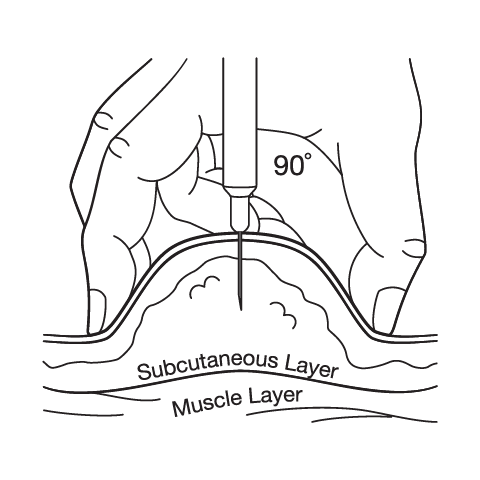
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Vitamin D3 (Cholecalciferol) Capsules
Vitamin D3 (Cholecalciferol) Capsules Vitamin B-Complex Injection
Vitamin B-Complex Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Methylcobalamin Injection (Vitamin B12)
Methylcobalamin Injection (Vitamin B12) BCAA Injection
BCAA Injection Bi-Amino Injection
Bi-Amino Injection Zinc Sulfate Injection
Zinc Sulfate Injection Coenzyme Q10 (Ubidecarenone) Injection
Coenzyme Q10 (Ubidecarenone) Injection