Product Overview
Bi-Est Cream is a compounded topical formulation combining estriol and estradiol in a 50:50 ratio OR 80:20 ratio, delivered in a smooth dermatological base that enables flexible strengths from 1 mg/mL up to 10 mg/mL across 30 mL dispensers; the preparation is dispensed only pursuant to an individualized prescription from a state-licensed 503A pharmacy and is intended for transdermal placement on thin skin areas for systemic menopausal symptom relief.[1]
Bioidentical hormone replacement emerged to mirror endogenous molecular structures after concerns about conjugated equine estrogens and synthetic progestins led to a decline in conventional therapy; estriol and estradiol are classified as bioidentical because their stereochemistry is identical to endogenous hormones, allowing physiologic receptor engagement while avoiding xenobiotic metabolites.[2]
Topical delivery bypasses first-pass hepatic metabolism, producing steadier serum estradiol-to-estrone ratios and lower triglyceride excursions than oral tablets, thereby potentially reducing thrombotic and lipid-mediated risks while permitting finer dose titration through variable cream concentrations.[3]
Estriol, although traditionally considered a “weaker” estrogen, demonstrates rapid dermal absorption and favorable distribution with lower affinity for sex-hormone-binding globulin, leading to proportionally higher free fractions that contribute to symptom relief despite modest receptor potency.[4]
Compounded preparations are formulated under United States Pharmacopeia non-sterile standards and state board oversight; unlike FDA-registered 503B outsourcing facilities, 503A pharmacies may tailor Bi-Est Cream to prescriber directions for individual patients, yet products remain unapproved by the FDA and rely on practitioner monitoring.[5]
A typical initiation regimen for symptomatic menopausal patients employs 0.5 mL of 1 mg/mL cream daily, delivering approximately 0.5 mg of combined estrogen; dose titration in 0.25-0.5 mL increments every 4-6 weeks is guided by clinical response and serum level assessments.[22]
Professional society consensus emphasizes individualized dosing, advocating the lowest effective amount to achieve symptom control while periodically re-evaluating need, adding cyclic or continuous progesterone in women with an intact uterus, and documenting informed consent regarding off-label compounded use.[23]
Upon dermal penetration, estradiol and estriol diffuse into systemic circulation and bind estrogen receptors α and β, forming ligand-receptor dimers that translocate to the nucleus and modulate transcription through estrogen response elements, thereby influencing gene networks related to vasomotor control, bone turnover, urogenital epithelium, and neurocognitive pathways.[6]
Estriol shows partial agonist behavior at ER-α and context-dependent antagonism in breast tissue, whereas estradiol exhibits full agonism; co-administration in equal ratios may balance proliferative signaling with competitive occupancy, providing symptom control while potentially attenuating unchecked mitogenic drive in estrogen-sensitive tissues.[7]
Dermal absorption follows partitioning through stratum corneum lipids; proprietary permeation-enhancing bases can increase flux rates, and in vitro models demonstrate measurable systemic levels within hours, with steady-state achieved after multiple applications.[8]
Once systemic, transdermal estradiol exerts favorable effects on arterial stiffness, C-reactive protein, and fibrinogen compared with oral routes, suggesting a mechanism rooted in avoidance of hepatic induction of clotting factors and maintenance of endothelial nitric oxide synthase activity.[9]
Alternative non-hepatic routes such as intranasal estradiol further validate that bypassing first-pass metabolism yields rapid symptom relief and predictable pharmacokinetics, underscoring the shared mechanistic theme of direct systemic entry.[10]
Active or prior venous thromboembolism, ischemic stroke, or inherited thrombophilias remain principal contraindications because estrogen up-regulates hepatic synthesis of coagulation proteins and down-regulates anticoagulant pathways, amplifying baseline thrombotic risk.[11]
Bi-Est Cream should be avoided in individuals with gallbladder disease or a history of cholelithiasis since estrogen increases biliary cholesterol saturation and gallbladder stasis, raising cholecystectomy rates in postmenopausal users.[12]
Migraines with aura and poorly controlled hypertension warrant caution or avoidance owing to estrogen-associated cerebral vasodilation, enhanced cortical excitability, and a documented elevation in ischemic stroke incidence among susceptible populations.[13]
Potent cytochrome P450 3A4 inducers such as rifampin, carbamazepine, and St. John’s wort accelerate estradiol metabolism, potentially leading to symptom breakthrough and necessitating dose reassessment or alternative therapy.[14]
Concurrent application of other estrogenic products or topical agents containing strong solvents may alter cutaneous permeability, heightening systemic exposure and increasing the likelihood of estrogen excess manifestations.[15]
Use in patients receiving aromatase inhibitors or selective estrogen receptor modulators for breast cancer should be avoided because exogenous estrogen counteracts therapeutic goals and may diminish oncologic efficacy.[16]
Common adverse events include breast tenderness, headache, vaginal discharge, and localized erythema at the application site; most are dose-dependent and respond to concentration adjustments or alternate sites of application.[17]
Systemic absorption from topical or vaginal routes can yield estradiol levels comparable to oral preparations; clinicians must monitor for endometrial proliferation, mastalgia, or fluid retention, particularly when therapy exceeds physiologic replacement ranges.[18]
Case reports describe new-onset or exacerbated breast discomfort within weeks of dose escalation; symptom resolution frequently follows dose reduction or incorporation of cyclic progestogen where a uterus is present.[19]
Topical estrogen therapy is contraindicated in pregnancy because exogenous estradiol exposure during organogenesis may disrupt sexual differentiation and is categorized as teratogenic; prescribers should verify non-pregnant status before initiation.[20]
Although low-dose vaginal preparations appear to confer minimal systemic exposure, measurable serum estradiol increases have been documented, and therapy during pregnancy or lactation is discouraged unless benefits clearly outweigh risks.[21]
Chemical stability studies indicate that compounded estrogen creams in pluronic-lecithin organogel or similar bases retain ≥ 90 % potency for at least 60 days; beyond-use dates should not exceed this interval unless validated stability data support extension.[24]
Store Bi-Est Cream at controlled room temperature (20-25 °C), protected from direct light and moisture; avoid refrigeration unless specified by the compounding pharmacist, and discard any unused portion after the assigned beyond-use date.[25]
- Childs, W. (2025, June 23). Biest (Medication) Guide for Menopause: Side Effects & Dosing. RestartMed. https://www.restartmed.com/biest/
- Holtorf, K. (2011). The bioidentical hormone debate: Are bioidentical hormones safer or more efficacious than commonly used synthetic versions? Mayo Clinic Proceedings, 86(7), 700-707. https://www.mayoclinicproceedings.org/article/S0025-6196(11)60072-4/fulltext
- Kuhl, H. (1987). Pharmacology and pharmacokinetics of estrogens. American Journal of Obstetrics and Gynecology, 156(4), 1289-1303. https://doi.org/10.1016/0002-9378(87)90166-9
- Nijland, M. J., et al. (2025). Serum concentrations of estriol vary widely after vaginal application. British Journal of Clinical Pharmacology. https://doi.org/10.1111/bcp.14635
- Trovall, R. (2025, May 1). What is a compounding pharmacy? Houston Chronicle. https://www.houstonchronicle.com/news/houston-texas/health/article/compounding-pharmacy-big-pharma-20281787.php
- .Björnström, L., & Sjöberg, M. (2005). Mechanisms of estrogen receptor signaling. Molecular Endocrinology, 19(4), 833-842. https://doi.org/10.1210/me.2004-0013
- Liu, Y., & Zhang, S. (2024). Estrogen receptor signaling and targets. Molecular Medicine Reports, 29(3), 13268. https://doi.org/10.3892/mmr.2024.13268
- Mitrogotri, S., et al. (2023). In vitro percutaneous absorption of compounded estradiol/estriol formulations. Journal of Investigative Dermatology. https://doi.org/10.1016/j.jid.2023.05.016
- Zhao, L., et al. (2025). Effects of transdermal estrogens with MPA on cardiovascular risk factors. Diabetes, Metabolic Syndrome and Obesity, 18, 596. https://doi.org/10.1186/s13098-025-01664-1
- Pita, R., et al. (1998). Intranasal 17β-oestradiol for menopausal symptoms. The Lancet, 351(9101), 922-927. https://doi.org/10.1016/S0140-6736(98)06196-0
- Rosendaal, F. R., & Helmerhorst, F. M. (2025). Estrogen and thrombosis: Bench to bedside review. Thrombosis and Haemostasis, 125(5), 887-899. https://www.simplevas.net/wp-content/uploads/2025/05/Estrogen-and-Thrombosis.pdf
- LaCroix, A. Z., et al. (2005). Effect of estrogen therapy on gallbladder disease. JAMA, 293(20), 2462-2469. https://jamanetwork.com/journals/jama/fullarticle/200193
- Loder, E., et al. (2021). The complex relationship between estrogen and migraines. Systematic Reviews, 10(1), 72. https://doi.org/10.1186/s13643-021-01618-4
- HelloPharmacist. (2024). Estradiol with rifampin interaction details. https://hellopharmacist.com/drug-interactions/drugs/estradiol/estradiol-with-rifampin
- Georgiadis, M., et al. (2025). Percutaneous absorption of permeation-enhancing estrogen formulations. Pharmaceuticals, 18(4), 596. https://doi.org/10.3390/ph18040596
- Eliassen, H., et al. (2025). Circulating estrogen metabolites and breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 34(3), 375-384. https://doi.org/10.1158/1055-9965.EPI-24-0577
- Drugs..com. (2024). Estradiol topical side effects. https://www.drugs.com/sfx/estradiol-topical-side-effects.html
- Notelovitz, M. (1978). Systemic absorption and sustained effects of vaginal estrogen creams. JAMA, 240(12), 1322-1324. https://doi.org/10.1001/jama.1978.03290120024017
- AntiGravity Wellness. (2025). Breast tenderness on estradiol. https://antigravitywellness.com/breast-tenderness-on-estradiol-heres-what-you-need-to-know/
- Feldkamp, M. L., et al. (2022). Prenatal exposure to teratogenic medications. American Journal of Obstetrics and Gynecology, 226(2), 213-222. https://doi.org/10.1016/j.ajog.2022.01.008
- Suckling, J., et al. (2005). Safety of vaginal oestrogen in postmenopausal women. The Obstetrician & Gynaecologist, 7(4), 241-246. https://doi.org/10.1576/toag.7.4.241.27118
- Menopause Method. (2021). Most common starting dose for Bi-Est creams. https://courses.menopausemethod.com/wp-content/uploads/2021/02/pp10h-267-276.pdf
- American College of Obstetricians and Gynecologists. (2023). Compounded bioidentical menopausal hormone therapy. https://www.acog.org/clinical/clinical-guidance/clinical-consensus/articles/2023/11/compounded-bioidentical-menopausal-hormone-therapy
- American Society of Health-System Pharmacists. (2022). The pharmacist guide to assigning a beyond use date. https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/compounding/docs/The-Pharmacist-Guide-to-Assigning-a-Beyond-Use-Date-_final.pdf
- United States Pharmacopeia. (2024). USP compounding standards and beyond-use dates. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf
- Lalonde, D. (2025). How should I store my hormone therapy products? Red Leaf Wellness. https://redleafwellness.ca/faq-items/how-should-i-store-my-hormone-therapy-products/
- .My Menopause Centre. (2023). Storing and disposing of HRT products. https://www.mymenopausecentre.com/gp-resources/storing-your-hormone-replacement-therapy-hrt-products/
- Appliance Update. (2025). Does estradiol valerate need refrigeration? https://applianceupdate.com/does-estradiol-valerate-need-to-be-refrigerated/
- Cabot, S. (2024). Hormone replacement therapy and the liver. Liver Doctor. https://www.liverdoctor.com/hormone-replacement-therapy-and-the-liver/
- Lee, H., & Chen, Y. (2023). Transdermal 17β-estradiol and cardiovascular risk: A meta-analysis. Maturitas, 175, 1-12. https://doi.org/10.1016/j.maturitas.2023.04.012
- U.S. Food and Drug Administration. (2016). Pharmacy compounding under section 503A. https://www.fda.gov/media/94393/download
- Barlow, G. (2023). Updates on 503A compounding and drug shortages. Pharmacy Times. https://www.pharmacytimes.com/view/updates-on-503a-compounding-the-impact-of-drug-shortages
- Shah, N., & Agarwal, G. (2015). Estrogen as a regulator of insulin action and mitochondrial function. International Journal of Endocrinology, 2015, 916585. https://doi.org/10.1155/2015/916585
- Schubert, C. (2023). Estrogen receptor. In Encyclopedia of Signaling Molecules (pp. 1-14). Springer. https://doi.org/10.1007/978-3-030-62345-6_5323
- Falkenstein, E., et al. (2006). Nongenomic actions of estrogens. Pharmacology & Therapeutics, 109(1-2), 58-74. https://doi.org/10.1016/j.pharmthera.2005.06.013
- Levin, E. R., et al. (2019). Estrogen receptor signaling mechanisms. Journal of Steroid Biochemistry and Molecular Biology, 191, 105364. https://doi.org/10.1016/j.jsbmb.2019.105364
What distinguishes a compounded cream from commercial patches?
Customized ratios and strengths permit fine-tuned therapy and excipient avoidance, enhancing adherence.[26]
Does the cream need refrigeration during travel?
Room-temperature stability testing supports ambient storage if extremes of heat over 30 °C and prolonged sunlight are avoided.[27]
Can I freeze the product to extend shelf-life?
Freezing may cause phase separation and inconsistent dosing; follow labeled beyond-use dating without freezing.[28]
Is liver disease a contraindication?
Moderate hepatic impairment requires caution because estrogen undergoes hepatic conjugation; clinicians should monitor enzymes and consider non-systemic options.[29]
Does transdermal estrogen raise cardiovascular risk the same way oral therapy does?
Meta-analytic data suggest transdermal routes exert neutral or favorable effects on blood pressure, lipids, and CRP compared with oral estrogens.[30]
What legal framework governs this medication?
Section 503A allows patient-specific compounding but exempts the product from FDA approval and Good Manufacturing Practice requirements.[31]
Will compounded estrogen remain available during drug shortages?
503A pharmacies may fill gaps when commercial products are scarce, though regulatory proposals could restrict certain hormones.[32]
Could this therapy influence insulin sensitivity?
Experimental work links estrogen signaling to improved mitochondrial function and glucose homeostasis, though clinical translation remains under study.[33]
Are there non-genomic effects I should know about?
Estrogen can rapidly activate membrane-bound receptors, modulating calcium fluxes and kinase cascades separately from gene transcription.[34]
How fast does the cream act?
Nongenomic vasomotor modulation may begin within minutes, while genomic effects on bone and lipid metabolism evolve over weeks.[35]
Is dose escalation limitless?
Receptor saturation and diminishing returns occur; doses beyond symptom control increase adverse-event risk without added benefit.[36]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
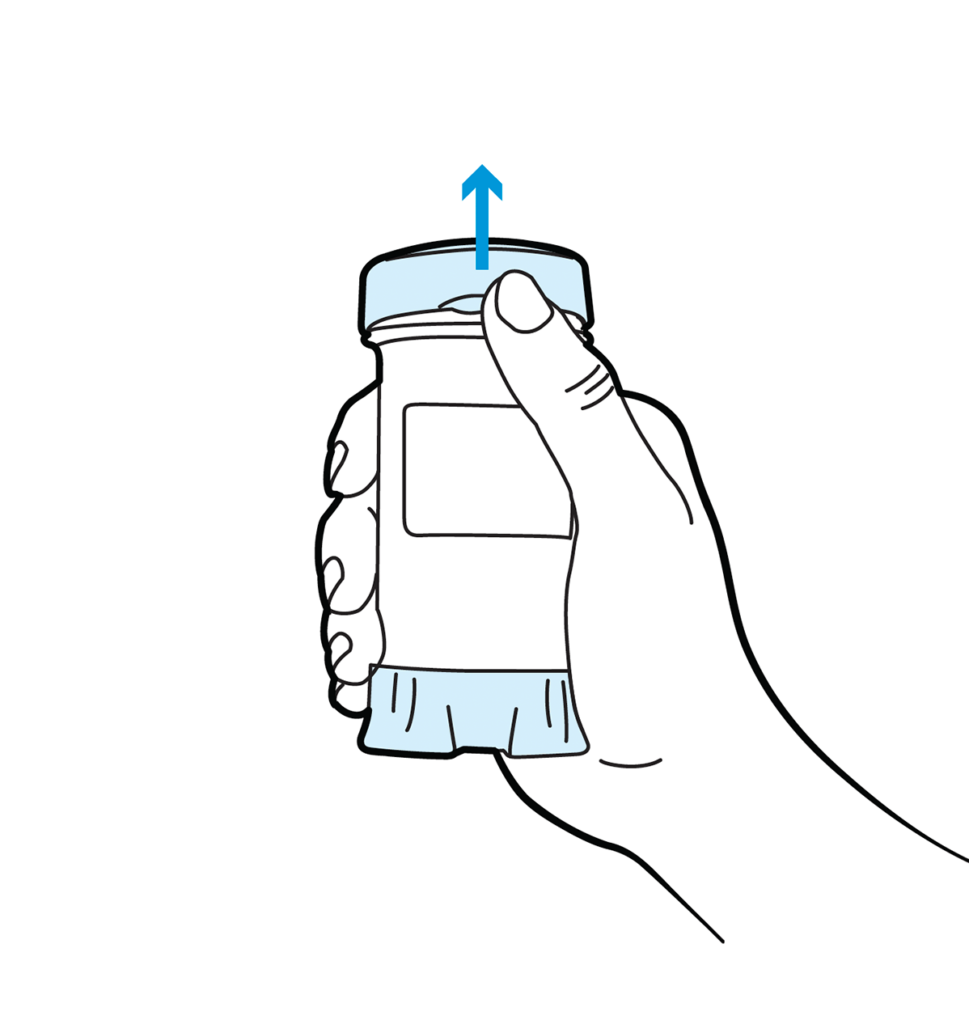
Topi-Click Dispensing Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.






 Estradiol Cypionate Injection
Estradiol Cypionate Injection Estradiol Capsules
Estradiol Capsules Estradiol Cream
Estradiol Cream Estradiol Patch
Estradiol Patch Progesterone Capsules
Progesterone Capsules Progesterone Cream
Progesterone Cream Testosterone Cream
Testosterone Cream DHEA Capsules
DHEA Capsules DHEA / Pregnenolone Capsules
DHEA / Pregnenolone Capsules