STORAGE REQUIREMENTS SHIFTING FOR SELECT MEDICATIONSLonger BUDs. Enhanced flexibility.
Certain medications now require refrigerated or frozen storage. This shift will support longer beyond-use dates (BUDs) while keeping the medications, ordering process, and shipping exactly the same.
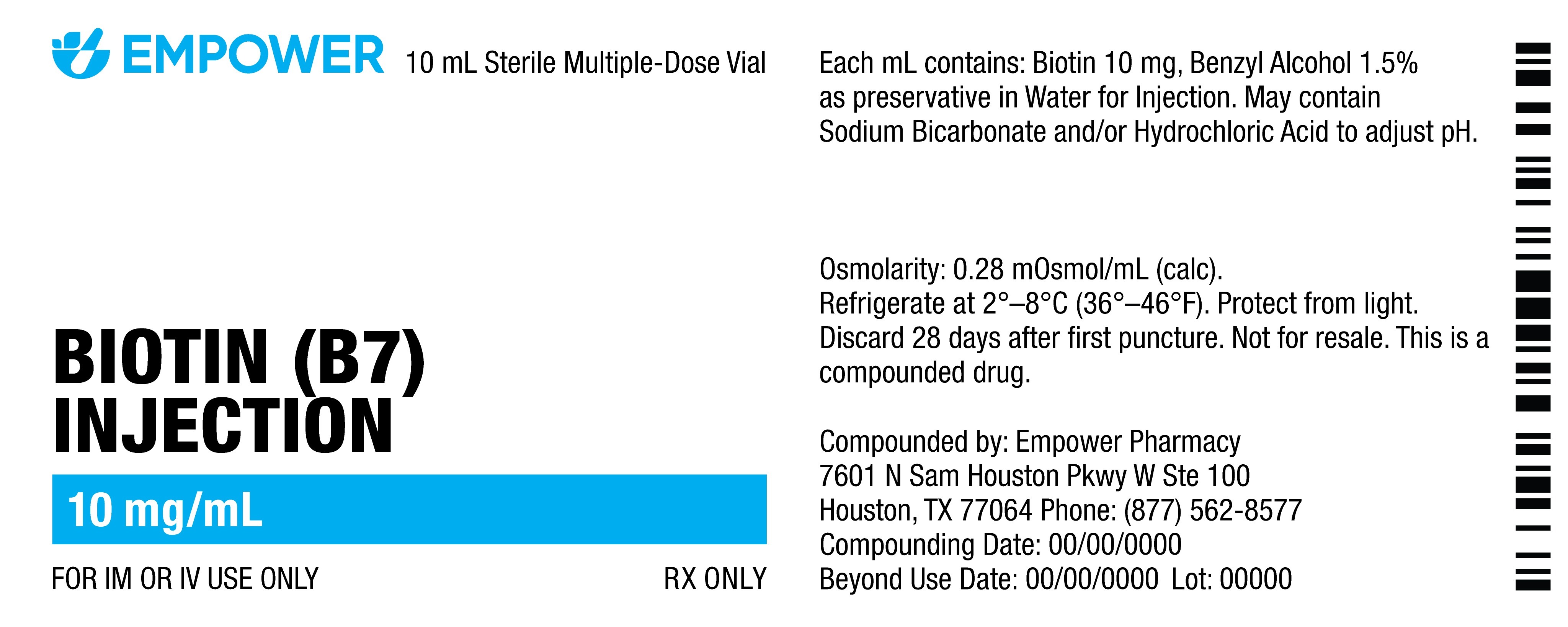
FROZEN Vs. REFRIGERATED VS. ROOM TEMPERATURE LABELS
These are examples of what medications requiring frozen, refrigerated or room temperature storage look like. The frozen label is on the left; the refrigerated label is in the middle; the room temperature label is on the right.


What You Need to Know
- No action required: This update is rolling out automatically.
- Shipping costs remain the same: Medications will continue to ship at room temperature, with no additional cost.
- Easy identification and adherence: Products to be refrigerated will be clearly labeled with light blue storage labels and include a package insert with instructions.
Additional Information
When is this change effective?
The first release of affected medications took place in February.
How will I know if I should refrigerate or freeze a medication?
There are several ways to know if a medication now requires refrigerated or frozen storage:
- Look at the label on the vial; it will always display storage instructions. Medications requiring refrigerated or frozen storage also have a light blue label. Medications requiring frozen storage will also have detailed temperature instructions in light blue text. Room temperature medications have a dark blue label.
- Look at the package. Products requiring refrigerated or frozen storage will be stamped on the outside package. Additionally, the box in which the vial arrives will have a bright green sticker that reads “OPEN IMMEDIATELY” on the front.
- Read the information sheet that is stapled to the medication package. It will detail the storage instructions required for your medication.
Do I need to store these medications in the refrigerator or freezer upon receipt?
Yes. Once customers receive their packages, they should immediately open the contents and place the product(s) in the refrigerator or freezer for its recommended storage condition. Customers can defer to the product label for storage requirements.
Lyophilized products that require frozen storage upon receipt will still require refrigerated storage after reconstitution.
For older lots, patients should continue to follow the storage instructions printed on the vial labels.
What if I still have questions about storing these medications?
Our team is here to help. Please call our Clinical staff with any additional questions you may have.
- Providers/Patients with Product-related questions: Call (877) 562-8577 (Option 3 > Option 2 > Option 2)
- Providers with Shipping or cost-related questions: Call (877) 562-8577 (Option 1 > Option 2 > Option 1)
- Patients with Shipping or cost-related questions: Call (877) 562-8577 (Option 1 > Option 1 > Option 1)
