Product Overview
† commercial product
Zinc Sulfate Injection is a sterile, preservative-free aqueous solution intended exclusively for intravenous use as a trace-element additive in parenteral nutrition formulations; each milliliter delivers ionized zinc that the body cannot synthesize and that is required to support hundreds of metallo-enzymes, nucleic-acid stability, and normal immune competence.[1]
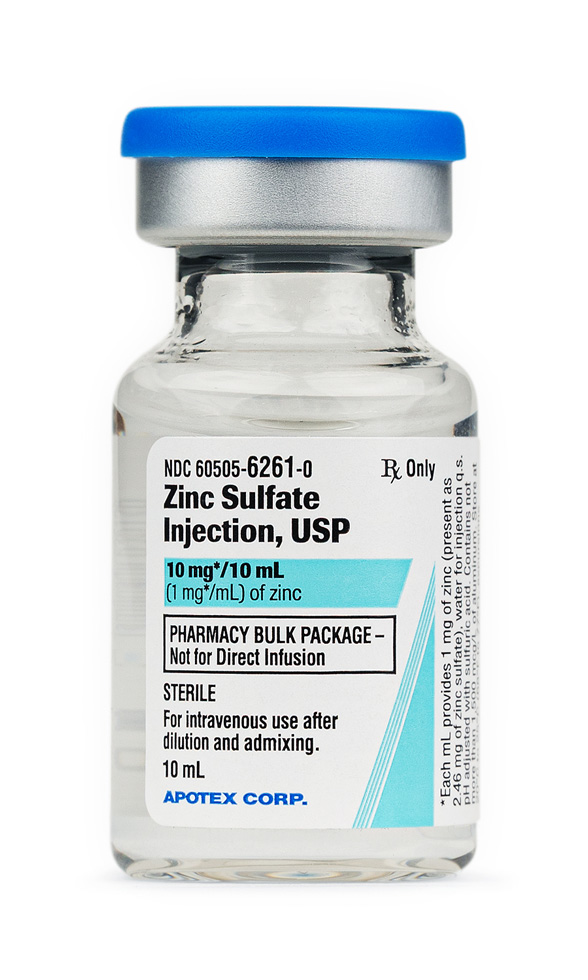
The product described here is compounded in a 503B outsourcing facility and supplied in two labeled concentrations: a 30 mL vial containing 10 mg zinc/mL for pharmacy bulk preparation and a 10 mL single-dose vial containing 1 mg zinc/mL that corresponds to a commercially manufactured presentation.[2]
According to current labeling, the solution is clear, colorless to pale straw, with a pH adjusted by sulfuric acid; it is designated Rx-only and must be diluted into a balanced amino acid/glucose admixture before infusion to avoid venous irritation or renal zinc wasting.[3]
The clinical rationale for parenteral zinc supplementation was established after total parenteral nutrition (TPN) became widespread; observational data showed that omission of zinc led to growth failure, hypogonadism, and the fatal acrodermatitis enteropathica phenotype in long-term recipients.[4]
Large reviews of trace-element physiology further demonstrate that zinc deficiency, even when subclinical, impairs wound healing, alters taste and smell, and predisposes to infectious complications, situating intravenous zinc as a core component of evidence-based nutrition support strategies.[5]
Guidelines recommend 2.5-4 mg elemental zinc/day for metabolically stable adults; doses may rise to 12 mg/day when gastrointestinal losses are high, and up to 300 µg/kg/day in premature neonates.[7][8]
For long-term home-PN, retrospective analyses suggest that a median dose of 7.6 mg/day maintained normozincemia in 90 % of adults, but individualized adjustments based on stool or fistula output were still required.[12]
Accidental 100-fold compounding errors have resulted in life-threatening cardiopulmonary collapse; management involved immediate cessation, whole-bowel irrigation, and chelation with calcium disodium EDTA, underscoring that automated compounding verification and barcode checks are mandatory safeguards.[13][14]
Zinc serves as a catalytic or structural co-factor for over 300 enzymes, including DNA and RNA polymerases, alkaline phosphatase, and superoxide dismutase; by binding to cysteine and histidine residues it stabilizes tertiary protein structure and modulates gene expression via zinc-finger motifs.[4][6]
Within the bloodstream, most infused zinc binds rapidly to albumin and α-2-macroglobulin; cellular uptake is then mediated by ZIP (Zrt/Irt-like Protein) importers and ZnT exporters whose coordinated activity maintains nanomolar cytosolic concentrations while permitting millimolar sequestration in secretory vesicles-regulation that is crucial during stresses such as burns or sepsis when requirements rise markedly.[7][8]
Once internalized, zinc acts as an anti-oxidant surrogate by inducing metallothionein, a cysteine-rich peptide that scavenges free radicals and buffers transition-metal toxicity; experimental models show that zinc depletion heightens oxidative DNA damage and increases permeability of epithelial barriers.[9]
Conversely, excessive intracellular zinc can initiate apoptosis through mitochondrial dysfunction and antagonize copper-dependent enzymes, underscoring the importance of precise dosing in parenteral formulations.[9]
Administration is contraindicated in patients with known hypersensitivity to zinc salts or any component of the formulation and in those with severe brittle anemia where additional zinc could impede copper utilization, as noted in labeling.[3]
Because the bulk-vial presentation contains detectable aluminum, prolonged use in premature neonates or individuals with impaired renal clearance warrants extreme caution to avoid cumulative neurotoxic exposure.[2]
Clinicians must also consider pre-existing low serum copper: repeated high-dose zinc infusions accelerate enteral metallothionein expression, sequestering dietary copper and precipitating secondary hypocupremia that manifests as anemia and neutropenia.[10]
Zinc competes with copper and iron for shared divalent-metal transporter-1 (DMT-1) pathways; pharmacodynamic antagonism is clinically relevant when high-dose zinc is combined with intravenous iron dextran or oral copper supplements, potentially producing reciprocal deficiencies.[10]
Compatibility data compiled by parenteral-nutrition societies indicate that zinc chelates with calcium and phosphate at concentrations exceeding customary PN ranges; meticulous order of mixing and maintenance of acidic pH (<6.0) prevent precipitation.[7]
Chelating agents such as penicillamine or ethylenediaminetetraacetic acid (EDTA) can markedly increase renal excretion of zinc, whereas corticosteroids may induce a negative zinc balance by promoting urinary losses, necessitating monitoring in long-term PN.[8]
Local infusion-site phlebitis can occur if the concentrate is administered undiluted or infused through peripheral veins; pain, erythema, and sclerosis have been described when concentrations exceed 1 mg zinc/mL.[3]
Systemic adverse reactions are uncommon at recommended doses but may include diaphoresis, dizziness, nausea, and, in extreme overdoses, hypotension and pulmonary edema attributable to direct endothelial injury.[10]
Labeling reports that fetal safety data are limited; therefore inadvertent maternal exposure beyond physiological requirements could, in theory, disturb embryonic copper metabolism, although controlled studies in humans are lacking.[11]
Prolonged parenteral supplementation without laboratory surveillance has occasionally led to hypozincemia (>200 µg/dL) with concomitant hypocupremia; cases resolved after dose reduction and copper repletion, emphasizing the need for monthly trace-element panels in stable home-PN patients.[12]
Pregnancy category C applies to intravenous zinc: animal studies are inadequate, and well-controlled human investigations are absent; nevertheless, parenteral doses within the Dietary Reference Intake (11-12 mg elemental zinc/day) are not expected to pose teratogenic risk, whereas severe maternal deficiency correlates with pre-term birth.[11]
When parenteral nutrition is indispensable during hyperemesis gravidarum or severe malabsorption, clinicians may cautiously titrate zinc sulfate to maintain serum levels between 70 and 120 µg/dL, balancing the essential role of zinc in neural-tube development against theoretical concerns of copper-zinc antagonism.[6]
Vials should be stored at controlled room temperature, 20 - 25 °C (68 - 77 °F); exposure to extreme heat may accelerate pH drift and precipitate insoluble zinc salts.[2]
After first puncture of a pharmacy bulk vial, the contents must be used in a certified cleanroom within 4 hours, then discarded; single-dose vials offer reduced contamination risk but cannot be admixed directly into solutions containing bicarbonate.[3]
- Drugs..com. (2025, June 18). Zinc sulfate injection: Package insert / prescribing information. https://www.drugs.com/pro/zinc-sulfate-injection.html
- American Regent, Inc. (2009). Concentrated zinc sulfate injection, USP [Prescribing Information]. https://americanregent.com/media/1858/zinc-sulfate-conc-prescribing-information.pdf
- DailyMed. (2023, August 31). Zinc sulfate injection, solution. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=74d8d07b-cfd9-4262-b689-fea12673bbd1
- Jeejeebhoy, K. (2009). Zinc: An essential trace element for parenteral nutrition. Gastroenterology, 137(5 Suppl), S7-S12. https://doi.org/10.1053/j.gastro.2009.08.014
- Stehle, P., Stoffel-Wagner, B., & Kuhn, K. S. (2016). Parenteral trace element provision: Recent clinical research and practical conclusions. European Journal of Clinical Nutrition, 70, 886-893. https://doi.org/10.1038/ejcn.2016.53
- Perks, P., Huynh, E., Kaluza, K., & Boullata, J. I. (2022). Advances in trace element supplementation for parenteral nutrition. Nutrients, 14(9), 1770. https://doi.org/10.3390/nu14091770
- American Society for Parenteral and Enteral Nutrition. (2019). Appropriate dosing for parenteral nutrition: ASPEN recommendations. https://www.nutritioncare.org/uploadedFiles/Documents/Guidelines_and_Clinical_Resources/PN%20Dosing%201-Sheet-FINAL.pdf
- Merck Manual Professional Version. (2025). Basic adult daily requirements for parenteral nutrition. https://www.merckmanuals.com/professional/multimedia/table/basic-adult-daily-requirements-for-parenteral-nutrition
- Agency for Toxic Substances and Disease Registry. (2020). Toxicological profile for zinc. https://www.atsdr.cdc.gov/toxprofiles/tp60-c3.pdf
- Agnew, U. M., & Slesinger, T. L. (2022). Zinc toxicity. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK554548/
- Drugs..com. (2024, June 12). Zinc sulfate: Pregnancy and breastfeeding warnings. https://www.drugs.com/pregnancy/zinc-sulfate.html
- Btaiche, I. F., Carver, P. L., & Welch, K. B. (2011). Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. Journal of Parenteral and Enteral Nutrition, 35(6), 736-747. https://doi.org/10.1177/0148607111413902
- Burkhart, K. K., Kulig, K. W., & Rumack, B. (1990). Whole-bowel irrigation as treatment for zinc sulfate overdose. Annals of Emergency Medicine, 19(10), 1167-1170. https://doi.org/10.1016/S0196-0644(05)81523-9
- Brocks, A., Reid, H., & Glazer, G. (1977). Acute intravenous zinc poisoning. British Medical Journal, 1(6073), 1390-1391. https://doi.org/10.1136/bmj.1.6073.1390
- Jin, J., Mulesa, L., & Carrilero Rouillet, M. (2017). Trace elements in parenteral nutrition: Considerations for the prescribing clinician. Nutrients, 9(5), 440. https://doi.org/10.3390/nu9050440
Does zinc sulfate injection contain preservatives?
Yes; the compounded formulation is preserved to reduce the risk of benzyl-alcohol toxicity in neonates.[4] The commercial version is preservative-free.[15]
Can it be infused peripherally?
Only after dilution to <1 mg/mL elemental zinc and with osmolarity compatible with peripheral veins.[10]
How soon do serum levels respond?
Plasma zinc corrects within 24 hours, but tissue repletion may take several days.[8]
What monitoring is required?
Monthly serum zinc and copper, plus more frequent checks if renal or gastrointestinal losses fluctuate.[12]
Is it safe in cholestasis?
Dose reduction may be necessary because hepatic excretion is diminished, and zinc can accumulate.[9]
Does it interact with antibiotics?
High systemic zinc can chelate with tetracyclines, reducing their antibacterial activity.[10]
Why add zinc separately rather than use a multi-trace product?
Individual dosing allows rapid adjustment for patients with unusually high or low requirements.[6]
What signs indicate zinc overdose?
Fatigue, neuropathy, gastrointestinal distress, and paradoxical copper-deficiency anemia.[10]
Can zinc correct taste alterations in cancer patients on PN?
Limited studies suggest improvement, but controlled trials are sparse.[5]
Does refrigeration extend shelf life?
No; refrigeration is neither necessary nor recommended and may cause crystallization.[2]
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Tralement Injection
Tralement Injection Selenium Injection
Selenium Injection Magnesium Chloride Injection
Magnesium Chloride Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection Pyridoxine HCl Injection
Pyridoxine HCl Injection Methylcobalamin Injection (Vitamin B12)
Methylcobalamin Injection (Vitamin B12) Calcium Gluconate Injection
Calcium Gluconate Injection Glutathione Injection
Glutathione Injection Ondansetron Injection
Ondansetron Injection