Product Overview
Vardenafil belongs to the phosphodiesterase type 5 (PDE5) inhibitors drug class, a class of drugs commonly indicated for treatment of erectile dysfunction (ED). It is a selective phosphodiesterase (PDE) type 5 inhibitor similar to sildenafil and tadalafil. This class of drugs does not inhibit prostaglandins as do some agents for treating ED (e.g., alprostadil). Vardenafil and tadalafil are more selective for PDE5 than PDE6, which is present in the retina. This leads to less visual adverse effects such as those reported in sildenafil-treated patients. The advantage of vardenafil may be that it achieves maximum plasma concentration sooner than sildenafil and tadalafil which may result in a faster onset of action. In an analysis of 580 patients, erections improved in 80% of men, and the ability to complete sexual intercourse with ejaculation was increased. Efficacy in treating diabetics and radical prostectomy patients has also been demonstrated. According to ED treatment guidelines, oral phosphodiesterase type 5 inhibitors (PDE5 inhibitor) are considered first-line therapy.[1] Vardenafil was approved by the FDA in August 2003 for erectile dysfunction. An orally disintegrating tablet was approved by the FDA in June 2010.
Vardenafil is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. Nitric oxide then activates the enzyme guanylate cyclase, which results in increased levels of cGMP. Cyclic guanosine monophosphate causes smooth muscle relaxation in the corpus cavernosum thereby allowing inflow of blood; the exact mechanism by which cGMP stimulates relaxation of smooth muscles has not been determined. Phosphodiesterase type 5 is responsible for degradation of cGMP in the corpus cavernosum. Vardenafil enhances the effect of NO by inhibiting PDE5, thereby raising concentrations of cGMP in the corpus cavernosum. Vardenafil has no direct relaxant effect on isolated human corpus cavernosum and, at recommended doses, has no effect in the absence of sexual stimulation. Vardenafil has a greater selectivity for PDE5 versus PDE6, an enzyme found in the retina and involved in phototransduction. Sildenafil, another PDE inhibitor, has a lower selectivity for PDE5 vs PDE6 and is associated with abnormalities related to color vision with higher doses or plasma concentrations of the drug.
Phosphodiesterase type 5 is also abundant in lung tissue and esophageal smooth muscle. Inhibition of PDE5 in lung tissue results in pulmonary vasodilation which can be effective in treating pulmonary hypertension. Inhibition of esophageal smooth muscle PDE5 can cause a marked reduction in esophageal motility as well as in lower esophageal sphincter (LES) tone. These effects may be beneficial in certain motor disorders involving the esophagus such as diffuse spasm, nutcracker esophagus, and hypertensive LES. However, the reduction in LES tone can worsen the symptoms of gastroesophageal reflux disease (GERD). Dyspepsia is one of the more common adverse reactions associated with PDE inhibitor therapy.
Your health care provider needs to know if you have any of these conditions: bleeding disorders, eye or vision problems, including a rare inherited eye disease called retinitis pigmentosa, anatomical deformation of the penis, Peyronie’s disease, or history of priapism (painful and prolonged erection), heart disease, angina, a history of heart attack, irregular heartbeats, or other heart problems, high or low blood pressure, history of blood diseases, like sickle cell anemia or leukemia, history of stomach bleeding, kidney disease, liver disease, stroke, an unusual or allergic reaction to vardenafil, other medicines, foods, dyes, or preservatives, pregnant or trying to get pregnant, breast-feeding.
Vardenafil is contraindicated in patients with a known hypersensitivity to any component of the tablet. The safety and efficacy of combinations of vardenafil with other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended.
The safe and effective use of vardenafil in combination with other agents for treating erectile dysfunction has not been studied. Therefore, the use of such combinations is not recommended.
Vardenafil is contraindicated in patients who are currently on nitrate/nitrite therapy. Consistent with its known effects on the nitric oxide/cGMP pathway, vardenafil may potentiate the hypotensive effects of organic nitrates and nitrites. Patients receiving nitrates in any form are not to receive vardenafil. This includes any patient who receives intermittent nitrate therapies. It is unknown if it is safe for patients to receive nitrates once vardenafil has been administered. A suitable time interval following vardenafil dosing for safe administration of nitrates or nitric oxide donors has not been determined.
Vardenafil tablets are not recommended in patients with severe hepatic disease (Child-Pugh class C) or end stage renal disease requiring dialysis (severe renal impairment or renal failure). There are no controlled clinical studies on the safety and efficacy of vardenafil in these patients; therefore, vardenafil use is not recommended until further information is available. Patients with moderate hepatic impairment require a reduction in the starting dose of the regular tablets and a lower maximum dosage (see Indications/Dosage). Patients with mild hepatic impairment or mild to moderate renal impairment do not require adjustments in the vardenafil regular tablet dosage. The concomitant use of certain potent hepatic cytochrome P450 3A4 inhibitors may result in a requirement to adjust the vardenafil dosage (see Dosage and Drug Interactions).[2] Vardenafil orally disintegrating tablets provide increased exposure as compared to the regular tablets; therefore, the orally disintegrating tablets should not be used in patients with moderate or severe hepatic disease (Child-Pugh class B or C) or in patients on hemodialysis. Patients who require lower doses of vardenafil should use the regular tablets.[3]
Lower starting doses of vardenafil regular tablets should be considered for geriatric patients (>= 65 years) because elderly patients have higher plasma concentrations than younger males (18-45 years) (see Indications/Dosage).[2] In phase III clinical trials of the regular tablets, 834 elderly patients participated and there was no difference in safety or effectiveness compared to younger patients.[2] In trials with the orally disintegrating tablets, the vardenafil AUC in elderly patients (>= 65 years) was increased by 39% and the Cmax was increased by 21% as compared to patients <= 45 years; however, no differences in safety and efficacy were observed between elderly patients and those < 65 years old in placebo-controlled trials.[3] Elderly patients may potentially have renal and hepatic impairment which can increase vardenafil plasma concentrations. Because higher plasma concentrations may increase the incidence of adverse reactions, the regular tablet starting dose should be reduced in these patients.[2] Patients who require lower doses of vardenafil should use the regular tablets and not the orally disintegrating tablets.[3]
There is a degree of cardiac risk associated with sexual activity; therefore, prescribers should evaluate the cardiovascular status of their patients prior to initiating any treatment for erectile dysfunction. Health care professionals should consider whether the individual would be adversely affected by vasodilatory events. In particular, caution should be used if vardenafil is prescribed in the following patient groups: patients who have suffered a myocardial infarction, stroke, or life-threatening cardiac arrhythmias in the last 6 months; patients with resting hypotension (BP < 90/50) or resting hypertension (BP > 170/110); patients with cardiac disease, severe heart failure or coronary artery disease (CAD) which causes unstable angina including those with left ventricular outflow obstruction (e.g., aortic stenosis and idiopathic hypertrophic subaortic stenosis). Based on recommendations for sildenafil by the American College of Cardiology, it is recommended that vardenafil be used with caution in the following: patients with active coronary ischemia who are not taking nitrates (e.g., positive exercise test for ischemia); patients with congestive heart failure and borderline low blood pressure and borderline low volume status; patients on a complicated, multidrug, antihypertensive program; and patients taking drugs that can prolong the half-life of vardenafil. Vardenafil is contraindicated in patients currently onnitrate/nitrite therapy. In a double-blind, crossover, single-dose study of patients with stable CAD, vardenafil did not cause any impairment in exercise capabilities at levels equivalent to or greater than that achieved during sexual intercourse.[4] The effects of vardenafil on QT prolongation were evaluated in 59 healthy males using moxifloxacin (400 mg) as an active control. Therapeutic (10 mg) and supratherapeutic (80 mg) doses of vardenafil produced similar increases in QTc interval (e.g., 4-6 msec calculated by individual QT correction) as moxifloxacin. When vardenafil (10 mg) was given with gatifloxacin (400 mg), an additive effect on the QT interval was observed.[2] The effect of vardenafil on the QT interval should be considered when prescribing the drug. The manufacturer recommends that vardenafil not be used in patients with congenital long QT syndrome and those taking Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic drugs. Further, use vardenafil with caution in patients with cardiac disease or other conditions that may increase the risk of QT prolongation including cardiac arrhythmias, heart failure, bradycardia, myocardial infarction, hypertension, coronary artery disease, hypomagnesemia, hypokalemia, hypocalcemia, or in patients receiving medications known to prolong the QT interval or cause electrolyte imbalances. Females, geriatric patients, patients with diabetes mellitus, thyroid disease, malnutrition, alcoholism, or hepatic disease may also be at increased risk for QT prolongation.[5][6][7][8][9][10]
Prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been associated with PDE5 inhibitor administration. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 hours, whether painful or not, should seek emergency medical attention. Vardenafil and other agents for the treatment of erectile dysfunction should be used with caution in patients with penile structural abnormality (such as angulation, cavernosal fibrosis or Peyronie’s disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell disease, leukemia, multiple myeloma, polycythemia, or history of priapism).[2][11]
Patients should be reminded that vardenafil offers no protection against sexually transmitted disease. Counseling of patients about protective measures, including the prevention of transmission of human immunodeficiency virus (HIV) infection, should be considered.
Use vardenafil cautiously in patients with pre-existing visual disturbance. Post-marketing reports of sudden vision loss have occurred with phosphodiesterase inhibitors, including vardenafil. Vision loss is attributed to a condition known as non-arteritic anterior ischemic optic neuropathy (NAION), where blood flow is blocked to the optic nerve. This can cause permanent loss of vision. Vardenafil use should be discontinued in the event of sudden loss of vision in one or both eyes. Vardenafil use is not recommended in patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa. A minority of patients with the inherited condition retinitis pigmentosa have genetic disorders of retinal phosphodiesterases. Vardenafil use is not recommended in these patients until further information is available.[2][3]
Vardenafil is not indicated for use in females. Vardenafil is classified as FDA pregnancy risk category B. There are no adequate and well-controlled trials of vardenafil in humans during pregnancy.[2][3]
Vardenafil is not indicated for use in females and is therefore not recommended during breast-feeding. It is not known if vardenafil is excreted in human breast milk; however, it is known that the drug is excreted into the milk of lactating rats at concentrations approximately 10-fold greater than found in the plasma.[2][3]
There is no known indication for the use of vardenafil in neonates, infants, or children. Vardenafil should not be prescribed to these populations.
Vardenafil should be used cautiously in patients with gastroesophageal reflux disease (GERD) or hiatal hernia associated with reflux esophagitis. Like sildenafil, vardenafil can possibly decrease the tone of the lower esophageal sphincter and inhibit esophageal motility.[1][2]
Vardenafil should be administered to patients with coagulopathy only after careful benefit vs. risk assessment. Vardenafil alone does not prolong the bleeding time nor does its use in combination with aspirin cause any additive prolongation of the bleeding time. However, vardenafil has not been studied or administered to patients with bleeding disorders or significant active peptic ulcer disease. Therefore administer to these patients after careful benefit-risk assessment.[2]
Patients with a sudden decrease or loss of hearing (hearing impairment) should stop taking vardenafil and seek prompt medical attention. Hearing loss, which may be accompanied by tinnitus and dizziness, has been reported in temporal association with the intake of PDE5 inhibitors, including vardenafil; however, it is unknown if the hearing loss is directly related to PDE5 inhibitors or to other factors.[2][3]
The vardenafil orally disintegrating tablets contain aspartame, which is a source of phenylalanine. This may be harmful for people with phenylketonuria. Each tablet contains 1.01 mg of phenylalanine.[3]
The vardenafil orally disintegrating tablets contain sorbitol. Patients with hereditary fructose intolerance should not take the orally disintegrating tablets.[3]
This list may not include all possible contraindications.
Side effects that you should report to your doctor or health care professional as soon as possible: allergic reactions like skin rash, itching or hives, swelling of the face, lips, or tongue, breathing problems, changes in hearing, changes in vision, chest pain, fast, irregular heartbeat, prolonged or painful erection, seizures.
Side effects that usually do not require medical attention (report to your doctor or health care professional if they continue or are bothersome): back pain, dizziness, flushing, headache, indigestion, muscle aches, nausea, stuffy or runny nose.
Flushing occurred in 11% of those receiving vardenafil film-coated tablets and in 7.6% of patients receiving orally disintegrating tablets. The incidence of flushing appears to increase as the dose increases. Anaphylactoid reactions (including laryngeal edema) occurred in less than 2% of patients. Other events occurring in < 2% of patients include allergic edema, facial edema (angioedema), pruritus, photosensitivity reaction, sweating (hyperhidrosis), erythema, and rash (unspecified). The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo.[2][3]
Headache occurred in 15% of those receiving vardenafil film-coated tablets and in 14.4% of those receiving the orally-disintegrating tablets. The incidence of headache appears to increase as the dose increases. Neurologic effects that occurred in less than 2% of patients included asthenia, hypertonia, hypesthesia, dysesthesia, sleep disorders, amnesia, seizures, and paresthesias. The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo. Postmarketing reports indicate that seizures and seizure recurrence have occurred in temporal association with vardenafil use. The incidence of these adverse events is unknown. Transient global amnesia has been reported during post-marketing use of the drug.[2][3]
Gastrointestinal (GI) adverse reactions occurring in at least 2% of patients taking vardenafil film-coated tablets and more frequently than placebo included dyspepsia (4% vs 1%) and nausea/vomiting (2% vs 1%). Dyspepsia also occurred in 2.8% of patients receiving the orally disintegrating tablets. GI effects that occurred in less than 2% of patients included abdominal pain, diarrhea, dysphagia, esophagitis, gastritis, gastroesophageal reflux, vomiting, and xerostomia. Dyspepsia and nausea appear to increase as the dose increases. The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo.[2][3]
Arthralgia, myalgia, increased creatine phosphokinase (CPK), and increased muscle tone and cramping (muscle cramps) have been reported in less than 2% of patients receiving vardenafil during clinical trials. Neck pain has been reported with similar frequency. During controlled and uncontrolled clinical trials, back pain was reported in 2% of patients receiving vardenafil. The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo.[2][3]
Dizziness occurred in 2% of those receiving vardenafil film-coated tablets and 2.3% of patients receiving orally disintegrating tablets. Dizziness has been associated with a sudden decrease in hearing. Centrally-mediated effects occurring in less than 2% of patients included insomnia, somnolence (drowsiness), and vertigo. The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo.[2][3]
During clinical trials, cardiovascular adverse reactions that occurred in less than 2% of patients treated with vardenafil included angina pectoris, chest pain (unspecified), hypertension, hypotension, myocardial ischemia, myocardial infarction, orthostatic hypotension, palpitations, syncope, ventricular tachyarrhythmias (ventricular tachycardia), and sinus tachycardia. The effects of vardenafil on blood pressure were evaluated using single 20 mg doses of vardenafil in patients with erectile dysfunction. Vardenafil caused a mean maximum decrease in supine blood pressure of 7 mm Hg systolic and 8 mm Hg diastolic (compared to placebo), accompanied by a mean maximum increase in heart rate of 4 beats per minute. The maximum decrease in blood pressure occurred between 1 and 4 hours after dosing. After multiple dosing, the effects of vardenafil on blood pressure were similar on Day 31 as on Day 1. Vardenafil may add to the hypotensive effects of antihypertensive agents.[2][3]
Respiratory conditions occurring in at least 2% of patients taking vardenafil film-coated tablets and more frequently than placebo included rhinitis (9% vs 3%), sinusitis (3% vs 1%), and flu-like syndrome (3% vs 2%). The incidence of rhinitis appears to increase as the dose increases. Nasal congestion occurred in 3.1% of patients receiving the orally disintegrating tablets. Respiratory-related effects which occurred in less than 2% of patients included dyspnea, sinus congestion, and pharyngitis. The discontinuation rate due to adverse reactions in placebo-controlled trials was 3.4% for vardenafil and 1.1% for placebo.[2][3]
During clinical trials, ejaculation dysfunction occurred in less than 2% of patients treated with vardenafil. Prolonged erections greater than 4 hours and priapism have been reported rarely with PDE5 inhibitors, including vardenafil.
Epistaxis occurred in less than 2% of patients receiving vardenafil in clinical trials.[2][3]
Phosphodiesterase inhibitors, such as vardenafil, inhibit PDE6 in retinal rods and cones, which are involved in phototransduction in the retina. Changes in color vision were reported in < 2% of patients and occurred as a result of PDE6 inhibition. In single dose studies, dose-related impairment of color discrimination (blue/green) as well as reductions in electroretinogram (ERG) b-wave amplitudes were noted; peak effects were noted near the time of peak plasma levels (approximately 1 hour after dosing). These effects diminished but were still present 6 hours after administration. In a single dose study of 25 healthy males, vardenafil 40 mg did not alter visual acuity, intraocular pressure, or funduscopic and slit lamp findings.[2] In an 8-week, multiple-dose, placebo-controlled clinical trial, clinically significant changes in retinal function did not occur as assessed by ERG amplitudes or the Farnsworth-Munsell 100-hue test. The trial was designed to detect retinal function changes that might occur in more than 10% of patients. Of 52 enrollees, 32 subjects completed the study. Two patients in the vardenafil group reported transient cyanopsia (objects appear blue). Other ophthalmic adverse reactions occurring in < 2% of patients receiving vardenafil include blurred vision, chromatopsia, conjunctivitis (increased redness of the eye), dim vision, glaucoma, ocular pain, photophobia, visual impairment, ocular hyperemia, increased intraocular pressure, and watery eyes (lacrimation). Post-marketing reports have included cases of visual disturbances including retinal vein occlusion, visual field defects, reduced visual acuity, and loss of vision (temporary or permanent).[2][3] Non-arteritic anterior ischemic optic neuropathy (NAION) has also been reported rarely in patients using phosphodiesterase type 5 (PDE5) inhibitors.[13][14][15][16] It is thought that the vasoconstrictive effect of phosphodiesterase inhibitors may decrease blood flow to the optic nerve, especially in patients with a low cup to disk ratio. Symptoms, such as blurred vision and loss of visual field in one or both eyes, are usually reported within 24 hours of use. Most, but not all, of these patients who reported this adverse effect had underlying anatomic or vascular risk factors for development of NAION. These risk factors include, but are not limited to: low cup to disc ratio (‘crowded disc’), age over 50 years, diabetes, hypertension, coronary artery disease, hyperlipidemia, and smoking. Additionally, two patients had retinal detachment and one patient had hypoplastic optic neuropathy.[13] It is not yet possible to determine if these adverse events are related directly to the use of PDE5 inhibitors, to the patient’s underlying vascular risk factors or anatomical defects, to a combination of these factors, or to other factors.
Adverse reactions affecting hearing or otic special senses and occurring in < 2% of patients in controlled clinical trials of vardenafil include hearing loss and tinnitus. In addition, 29 reports of sudden changes in hearing including hearing loss or decrease in hearing, usually in 1 ear only, have been reported to the FDA during post-marketing surveillance in patients taking sildenafil, tadalafil, or vardenafil; the reports are associated with a strong temporal relationship to the dosing of these agents. Many times, the hearing changes are accompanied by vestibular effects including dizziness, tinnitus, and vertigo. Follow-up has been limited in many of the reports; however, in approximately one-third of the patients, the hearing loss was temporary. Concomitant medical conditions or patient factors may play a role, although risk factors for the onset of sudden hearing loss have not been identified. Patients should be instructed to promptly contact their physician if they experience changes in hearing.[2][3]
The effects of vardenafil on QT prolongation were evaluated in 59 healthy males using moxifloxacin (400 mg) as an active control. Therapeutic (10 mg) and supratherapeutic (80 mg) doses of vardenafil produced similar increases in QTc interval (e.g., 4-6 msec calculated by individual QT correction) as moxifloxacin (7 msec). The potential effect of vardenafil on the QT interval should be considered when prescribing the drug; the manufacturer recommends against drug use in certain patient groups with risk factors for QT prolongation (see Precautions).[2][3]
Changes in laboratory values have occurred infrequently. During controlled and uncontrolled clinical trials of vardenafil in over 4430 men (mean age 56, range 18-89 years), increased creatine kinase was reported in 2% of those receiving vardenafil versus 1% of those in the placebo group. Increased GGTP and elevated hepatic enzymes (e.g., abnormal liver function tests) were reported in less than 2% of patients.[2][3]
This list may not include all possible adverse reactions or side effects. Call your health care provider immediately if you are experiencing any signs of an allergic reaction: skin rash, itching or hives, swelling of the face, lips, or tongue, blue tint to skin, chest tightness, pain, difficulty breathing, wheezing, dizziness, red, a swollen painful area/areas on the leg.
Vardenafil is not indicated for use in females. Vardenafil is classified as FDA pregnancy risk category B. There are no adequate and well-controlled trials of vardenafil in humans during pregnancy.[2][3]
Vardenafil is not indicated for use in females and is therefore not recommended during breastfeeding. It is not known if vardenafil is excreted in human breastmilk; however, it is known that the drug is excreted into the milk of lactating rats at concentrations approximately 10-fold greater than found in the plasma.[2][3]
Store this medication in its original container at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the beyond use date. Do not flush unused medications or pour down a sink or drain.
- Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol 2005;174:230-9.
- Levitra (vardenafil) package insert. Kenilworth, NJ: Schering-Plough; 2007 Mar.
- Staxyn (vardenafil orally disintegrating tablets) package insert. Whitehouse Station, NJ: Schering-Plough; 2010 Jun.
- Thadani U, Smith W, Nash S, et al. The effect of vardenafil, a potent and highly selective phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction, on the cardiovascular response to exercise in patients with coronary artery disease. J Am C
- Roden, DM. Drug-induced prolongation of the QT interval. New Engl J Med 2004;350:1013-22.
- Crouch MA, Limon L, Cassano AT. Clinical relevance and management of drug-related QT interval prolongation. Pharmacotherapy 2003;23:881-908.
- van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 2010;70(1):16-23.
- Benoit SR, Mendelsohn AB, Nourjah P, et al. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005;12(4):363-368.
- Koide T, Ozeki K, Kaihara S, et al. Etiology of QT prolongation and T wave changes in chronic alcoholism. Jpn Heart J 1981;22:151-166.
- Galli-Tsinopoulou A, Chatzidimitriou A, Kyrgios I, et al. Children and adolescents with type 1 diabetes mellitus have a sixfold greater risk for prolonged QTc interval. J Pediatr Endocrinol Metab 2014;27:237-243.
- Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin N Am 2007;34:631-642.
- Bortolotti M, Mari C, Giovannini M, et al. Effects of sildenafil on esophageal motility of normal subjects. Dig Dis Sci 2001;46:2301-2306.
- Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (Viagra): a report of seven new cases. J Neuroophthalmol 2005;25:9-13.
- Escaravage GK Jr, Wright JD Jr, Givre SJ. Tadalafil associated with anterior ischemic optic neuropathy. Arch Ophthalmol 2005;123(3):399-400.
- Bollinger K, Lee MS. Recurrent visual field defect and ischemic optic neuropathy associated with tadalafil rechallenge. Arch Ophthalmol 2005;123(3):400-1.
- Peter NM, Singh MV, Fox PD. Tadalafil-associated anterior ischaemic optic neuropathy. Eye 2005;19(6):715-7.
Administration Instructions
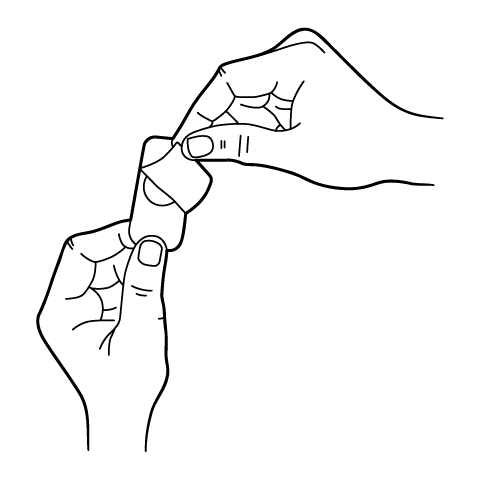
ODT and Troches Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Vardenafil / Apomorphine HCl Troches
Vardenafil / Apomorphine HCl Troches Vardenafil ODT
Vardenafil ODT Tadalafil Troches
Tadalafil Troches Tadalafil ODT
Tadalafil ODT Sildenafil ODT
Sildenafil ODT Enclomiphene Citrate Capsules
Enclomiphene Citrate Capsules Testosterone Nasal Gel
Testosterone Nasal Gel Testosterone Cypionate Injection
Testosterone Cypionate Injection Testosterone Enanthate Injection
Testosterone Enanthate Injection Testosterone Cypionate / Testosterone Propionate Injection
Testosterone Cypionate / Testosterone Propionate Injection