Testosterone Cypionate / Testosterone Propionate Injection
Product Overview
Testosterone Cypionate / Testosterone Propionate Injection is a compounded injectable formulation containing two testosterone ester forms, testosterone cypionate and testosterone propionate, dissolved in grapeseed oil. It is prepared by a licensed compounding pharmacy under Section 503A of the U.S. Food, Drug, and Cosmetic Act, meaning it is made for individual patients pursuant to a prescription and has not undergone FDA approval or pre-market evaluation of safety or effectiveness.[1] This medication is intended for use as testosterone replacement therapy in males with confirmed hypogonadism or markedly low testosterone levels due to conditions associated with a deficiency or absence of endogenous testosterone.[2] By combining a long-acting ester (cypionate) with a short-acting ester (propionate), the formulation is designed to provide both an initial increase in testosterone and a sustained release, potentially helping to maintain more stable hormone levels between doses. Like other testosterone products, it is classified as a Schedule III controlled substance in the United States due to its potential for abuse and misuse.[3]
As a compounded drug, this combination is available only through specialty pharmacies and requires a valid prescription from a licensed healthcare provider. It is not an FDA-approved commercially marketed product, and its use is generally reserved for patients whose medical needs cannot be met by standard FDA-approved therapies. The injection is typically prescribed to adult male patients who have clinical symptoms of testosterone deficiency and consistently low serum testosterone concentrations confirmed by laboratory testing. It may also be considered (with caution and specialist oversight) in certain adolescent cases of delayed puberty to induce the development of secondary sexual characteristics. However, this therapy is not indicated for men with age-related declines in testosterone alone, and its safety and efficacy have not been established for so-called “late-onset” hypogonadism in older males.[4] Likewise, it is not intended for use in healthy individuals for bodybuilding, athletic performance enhancement, or any non-medical purpose.
Because this product is a 503A compounded medication, each vial is made in limited quantities for a specific patient. The formulation described here provides 160 mg/mL of testosterone cypionate and 40 mg/mL of testosterone propionate (a total of 200 mg of testosterone per mL) in a sterile oil vehicle. It is administered by subcutaneous or intramuscular injection (often into the gluteal muscle) on a regimen determined by the treating physician to maintain physiologic testosterone levels. Therapy with this injectable should be initiated and monitored by qualified healthcare professionals, with appropriate follow-up to adjust dosing and ensure clinical efficacy and safety for each individual patient.
The dosage of testosterone cypionate/propionate injection is individualized to each patient’s needs. A common regimen for testosterone replacement in hypogonadal adult males is in the range of 50-100 mg of testosterone equivalent per week, administered via intramuscular injection. Some protocols use 150-250 mg given every 2 weeks instead, but more frequent dosing (such as weekly injections) is often preferred to maintain steadier hormone levels.[15] The presence of the shorter-acting propionate ester in this compound may provide a relatively rapid onset of effect, but it still requires regular ongoing dosing to sustain testosterone levels. Treatment typically begins at a conservative dose, and the amount and frequency are adjusted based on the patient’s serum testosterone measurements and clinical response. In all cases, therapy should be guided by a qualified physician.
Testosterone cypionate and testosterone propionate are esterified derivatives of the natural androgen testosterone. After intramuscular administration, these testosterone esters are absorbed gradually from the muscle and then enzymatically cleaved to release free testosterone. The two esters have different pharmacokinetic profiles: testosterone propionate is a short-chain ester that reaches peak levels quickly but has a relatively short elimination half-life (on the order of 1-2 days), necessitating more frequent dosing (e.g., every 2-3 days) to maintain stable levels. In contrast, testosterone cypionate has a longer half-life of approximately 8 days, allowing it to provide a sustained testosterone release over a week or more.[5] Once in circulation, a portion of the free testosterone is converted to dihydrotestosterone (DHT) by 5α-reductase in certain tissues, and both testosterone and DHT bind to intracellular androgen receptors. The hormone-receptor complex then acts as a transcription factor in the nucleus, altering gene expression in target cells to produce androgenic effects such as increased protein synthesis and nitrogen retention in muscle and bone.
Exogenous testosterone also exerts negative feedback on the hypothalamic-pituitary-gonadal axis: it suppresses pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to reduced endogenous testosterone production and diminished spermatogenesis. This mechanism explains why testosterone therapy may cause testicular atrophy, and decreased sperm counts during treatment. On the other hand, androgens are responsible for the normal development and maintenance of male secondary sexual characteristics, including the growth of the prostate and seminal vesicles, maturation of the penis and scrotum, the development of facial, chest, and axillary hair, deepening of the voice, and changes in muscle and fat distribution, which is the desired therapeutic outcome of replacement therapy.[8]
Testosterone cypionate/propionate injection is contraindicated in individuals with hypersensitivity to testosterone or any of the formulation’s components, and in males with carcinoma of the breast or known or suspected carcinoma of the prostate. This therapy must not be used in women who are pregnant or may become pregnant, due to the risk of serious fetal harm (androgen exposure is teratogenic and can cause virilization of a female fetus). Patients with severe cardiac, hepatic, or renal disease should also avoid testosterone injections, as exogenous androgens may exacerbate edema and other complications in these conditions.[10] Use of this medication is generally limited to the specific indications noted; it is not intended for patients without hypogonadism, and it is not indicated for enhancing athletic performance or for use in women (except in rare clinical circumstances under specialist supervision).
Concurrent use of testosterone with certain medications may require caution and monitoring. Androgens may increase the sensitivity to oral anticoagulants such as warfarin, potentially enhancing their blood-thinning effect and raising bleeding risk. If testosterone therapy is started in a patient on a blood thinner, more frequent monitoring of coagulation (e.g., INR) and dose adjustments of the anticoagulant may be necessary. Testosterone can also alter insulin or oral diabetic medication requirements: it may improve insulin sensitivity or otherwise lower blood glucose, so diabetic patients on insulin or hypoglycemic drugs might need their dosages reduced to avoid hypoglycemia. Additionally, the concurrent administration of corticosteroids (or ACTH) and testosterone can increase the likelihood of fluid retention and edema, which is particularly concerning in patients with heart failure or advanced liver disease.[11]
It is also reported that androgens can elevate the serum levels of certain drugs; for example, concurrent testosterone administration has been observed to increase oxyphenbutazone concentrations.[11] Apart from drug-drug interactions, anabolic steroids may affect laboratory tests: testosterone therapy can decrease levels of thyroxine-binding globulin, resulting in lower total T₄ (thyroxine) readings and higher T₃ uptake, although free thyroid hormone levels remain unchanged and clinical hypothyroidism does not occur.
Like any androgen therapy, testosterone injections can produce a range of side effects. Common adverse effects are generally mild and may include injection site reactions (pain, redness, or swelling at the injection area), acne or oily skin, increased body or facial hair growth (or conversely, accelerated male-pattern baldness in those predisposed), and breast tenderness or gynecomastia (enlargement of breast tissue in males).[12] Some patients report alterations in mood or libido (which can manifest as irritability, aggression, or changes in sex drive), as well as headache, fatigue, or changes in energy level.[12] If any mild side effects are persistent or troublesome, patients should inform their healthcare provider.
More serious or long-term side effects are possible as well. Testosterone therapy can increase red blood cell production, which in some cases leads to polycythemia (an abnormally high hematocrit); this can thicken the blood and heighten the risk of blood clots, so periodic blood counts are recommended. There is ongoing debate about cardiovascular risks: some studies have suggested that testosterone treatment might be associated with a higher incidence of heart attack or stroke in older men, though definitive evidence is inconclusive. Nevertheless, caution is advised in patients with a history of cardiovascular disease. Testosterone can also cause edema (fluid retention), especially in those with heart, liver, or kidney impairment, potentially worsening conditions like congestive heart failure. Venous thromboembolism (blood clots in the veins, such as deep vein thrombosis or pulmonary embolism) has been reported in users of androgen products and is considered a possible risk; patients should seek medical attention if they experience leg pain, swelling, sudden chest pain, or shortness of breath. In addition, high-dose or prolonged anabolic steroid use has been linked to hepatic abnormalities, including rare cases of benign liver tumors or hepatic peliosis, and even malignant liver tumors, although such effects are uncommon with the doses used in therapeutic testosterone replacement.[13] Finally, misuse or abuse of testosterone (at supra-physiologic doses) can lead to serious adverse outcomes affecting the heart, liver, and mental health.[13]
Testosterone cypionate/propionate injection is contraindicated in pregnancy. Women who are pregnant or may become pregnant must not use this medication, because exposure to exogenous testosterone can cause serious harm to the developing fetus. Testosterone is known to be teratogenic (disturbing fetal development) and can induce virilization of a female fetus (development of male characteristics in a genetic female).[14] If this drug is used during pregnancy, or if a patient becomes pregnant while receiving it, the patient should be informed of the potential hazard to the fetus.
Furthermore, because of the possibility of adverse effects on a nursing infant, this therapy is generally avoided in women who are breastfeeding.
This compounded injection should be stored at controlled room temperature (approximately 20-25 °C) and protected from light and excessive heat or moisture.[16] Do not refrigerate or freeze the vial.[16] The medication should remain in its original container until use, and any unused portion beyond the recommended beyond-use date should be safely disposed of. As with all medications, it must be kept out of reach of children.
- U.S. Food and Drug Administration (FDA). (n.d.). Compounding and the FDA: Questions and Answers. Retrieved June 14, 2025, from https://www.fda.gov/drugs/human-drug-compounding/compounding-and-fda-questions-and-answers
- MedlinePlus. (2025). Testosterone injection (AHFS Patient Medication Information). National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html
- Physicians Total Care, Inc. (2012). Testosterone cypionate injection, USP [Package insert]. DailyMed. Retrieved from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a34023dc-fc54-4f66-bab0-9596502c23a3
- University of Illinois Chicago, College of Pharmacy. (2024a). Testosterone cypionate (injectable solution): Side effects, dosage, uses, and more. Healthline. Retrieved from https://www.healthline.com/health/drugs/testosterone-cypionate-injectable-solution
- Drugs..com. (2025a). Testosterone cypionate injection - Prescribing information. Retrieved from https://www.drugs.com/pro/testosterone-cypionate.html
- Chu, K. Y., Kulandavelu, S., Masterson, T. A., Ibrahim, E., Arora, H., & Ramasamy, R. (2020). Short-acting testosterone appears to have lesser effect on male reproductive potential compared to long-acting testosterone in mice. F&S Science, 1(1), 46-52. https://doi.org/10.1016/j.xfss.2020.03.002
- Pfizer, Inc. (2018a). Depo-Testosterone® (testosterone cypionate) injection [Prescribing information]. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/085635s040lbl.pdf
- Pfizer, Inc. (2018b). Depo-Testosterone® (testosterone cypionate) injection - Warnings and precautions. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/085635s040lbl.pdf
- Pfizer, Inc. (2018c). Depo-Testosterone® (testosterone cypionate) injection - Clinical pharmacology. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/085635s040lbl.pdf
- Pfizer, Inc. (2018d). Depo-Testosterone® (testosterone cypionate) injection - Contraindications. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/085635s040lbl.pdf
- University of Illinois Chicago, College of Pharmacy. (2024b). Testosterone cypionate: Warnings and interactions. Healthline. Retrieved from https://www.healthline.com/health/drugs/testosterone-cypionate-injectable-solution#interactions
- MedlinePlus. (2025a). Testosterone injection - Side effects. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html#side-effects
- Drugs..com. (2025b). Testosterone cypionate - Hepatic effects (warning). Retrieved from https://www.drugs.com/pro/testosterone-cypionate.html
- Drugs..com. (2025c). Testosterone cypionate - Use in pregnancy. Retrieved from https://www.drugs.com/pro/testosterone-cypionate.html
- BasicMedicalKey. (n.d.). Gonadal function. Retrieved June 14, 2025, from https://basicmedicalkey.com/gonadal-function-2/
- MedlinePlus. (2025b). Testosterone injection - Storage and disposal. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html#storage
- Defy Medical. (2020a). Testosterone Cypionate/Propionate Blend (160/40 mg/mL) - Product description. Retrieved from https://www.defymedicalstore.com/013
- Defy Medical. (2020b). Testosterone Cypionate/Propionate Blend - Pharmacology. Retrieved from https://www.defymedicalstore.com/013
- MedlinePlus. (2025c). Testosterone injection - Administration instructions. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html
- Gulzar, A. (2025). Comparing testosterone cypionate vs. testosterone enanthate. Verywell Health. Retrieved from https://www.verywellhealth.com/testosterone-cypionate-vs-enanthate-8698016
- Mayo Clinic. (n.d.-a). Testosterone cypionate (intramuscular route) - Precautions. Retrieved June 14, 2025, from https://www.mayoclinic.org/drugs-supplements/testosterone-cypionate-intramuscular-route/precautions/drg-20563731
- MedlinePlus. (2017). Testosterone injection - Important warning. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html#warnings
- U.S. Food and Drug Administration (FDA). (2018). Prescription requirement under Section 503A of the FD&C Act (Guidance for Industry). Retrieved from https://www.fda.gov/media/112423/download
- MedlinePlus. (2025d). Testosterone injection - Controlled substance status. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html
- University of Illinois Chicago, College of Pharmacy. (2024c). How quickly does testosterone cypionate work? Healthline. Retrieved from https://www.healthline.com/health/drugs/testosterone-cypionate-injectable-solution
- MedlinePlus. (2025e). Testosterone injection - Fertility precautions. National Library of Medicine. Retrieved from https://medlineplus.gov/druginfo/meds/a614041.html#precautions
What is Testosterone Cypionate/Propionate injection used for?
It is used as a testosterone replacement therapy for men with low testosterone levels (male hypogonadism).[17]
Why does this injection contain two different forms of testosterone?
Combining a fast-acting testosterone propionate with a longer-acting testosterone cypionate provides both an immediate effect and a sustained release, helping to potentially maintain stable hormone levels.[18]
How is it administered, and how often?
It is given by intramuscular injection (usually into a large muscle like the buttock) at intervals determined by the doctor, commonly once every 1 to 2 weeks.[19]
What are the common side effects?
Possible side effects include injection site pain or swelling, acne or oily skin, increased body hair or hair loss, headache, changes in libido, and breast tenderness (gynecomastia).[20]
Are there serious side effects?
Serious adverse effects are uncommon but can include blood clots (for example, in the legs or lungs), high red blood cell count, swelling due to fluid retention, liver problems, or effects on the heart (especially in older men).[21]
Can women use this medication?
No, it is generally intended for men. Testosterone can cause virilization (development of male characteristics) in women and is contraindicated during pregnancy due to risk of birth defects.[22]
Is it FDA-approved?
No. This is a compounded medication prepared in a pharmacy under federal compounding laws, and it has not been evaluated or approved by the FDA.[23]
Do I need a prescription for this injection?
Yes. Testosterone is a Schedule III controlled substance in the US and is only available with a valid doctor’s prescription.[24]
How long will it take to see results from therapy?
The hormone starts acting quickly, but symptom improvements (such as in energy or libido) may take a few weeks; many patients begin to feel changes after about 3-4 weeks of regular injections.[25]
Will this treatment affect my fertility?
It might. Testosterone therapy can reduce sperm production during use, potentially lowering a man’s fertility. This effect is usually reversible after stopping treatment, but men who wish to have children should discuss it with their doctor.[26]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
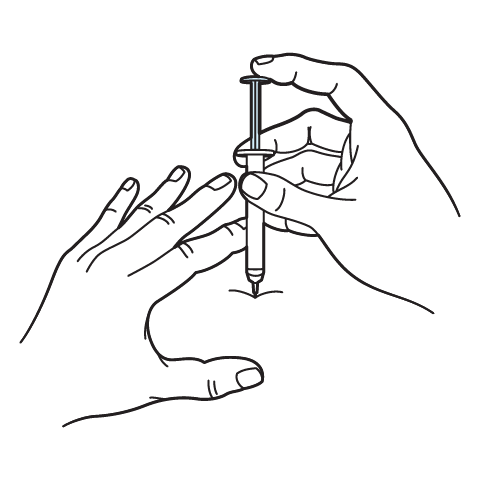
Intramuscular Injection Instructions
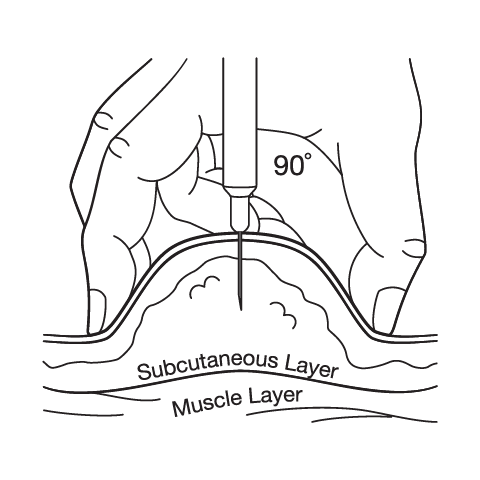
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Testosterone Cypionate Injection
Testosterone Cypionate Injection Testosterone Cream
Testosterone Cream Anastrozole Capsules
Anastrozole Capsules Enclomiphene Citrate Capsules
Enclomiphene Citrate Capsules HCG Injection
HCG Injection Sermorelin Acetate Injection
Sermorelin Acetate Injection