Product Overview
† commercial product
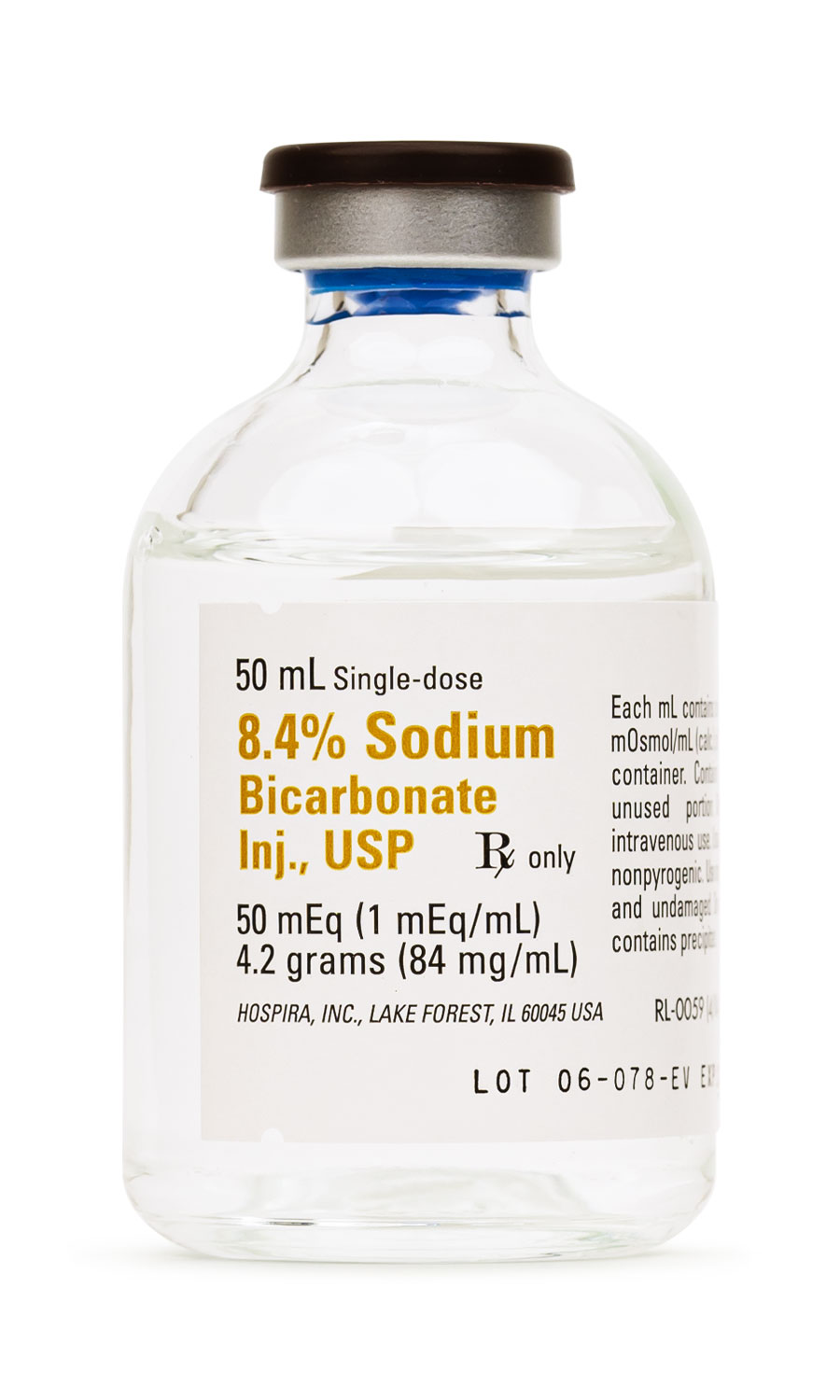
Sodium Bicarbonate Injection is an aqueous, hypertonic, preservative-free formulation of sodium bicarbonate intended for intravenous administration in settings where rapid correction of severe metabolic acidosis, life-threatening hyperkalemia, certain drug toxicities, or cardiopulmonary arrest is judged clinically necessary. The 8.4 % solution supplied in a 50 mL single-dose vial contains 1 mEq of sodium and bicarbonate per milliliter, yielding an osmolality in excess of 2000 mOsm/kg; this high osmotic load requires central venous delivery or very slow peripheral infusion to reduce the risks of chemical phlebitis and tissue necrosis. Because it confers both sodium and alkali, the product may rapidly raise serum pH, restore buffering capacity, and transiently shift extracellular potassium into cells, yet these benefits must be balanced against the potential for hypernatremia, volume overload, reduced tissue oxygen release, and paradoxical intracellular acidosis if ventilation is inadequate. The solution is manufactured under current good manufacturing practices at a registered 503B outsourcing facility and is dispensed by prescription only for use by qualified healthcare professionals[1].
Although sodium bicarbonate has been employed in clinical medicine for more than a century, contemporary guidelines urge a judicious, physiology-guided approach rather than routine empirical use. Large observational studies in critical care populations suggest that indiscriminate infusion fails to improve-and may even worsen-mortality except in carefully selected subgroups such as patients with profound acidemia (arterial pH ≤ 7.1) combined with acute kidney injury or drug-induced sodium channel blockade. Prescribers are therefore encouraged to base dosing on calculated base deficit, ongoing acid production, renal function, fluid status, and concomitant electrolyte disturbances, while monitoring arterial blood gases and chemistry at frequent intervals to avoid overshoot alkalosis[2].
Individual dosing is guided by the formula: bicarbonate required (mEq) = 0.2 × body weight (kg) × base deficit, administered slowly and reassessed with serial blood gases. Typical adult regimens for moderate metabolic acidosis employ 50 - 150 mEq diluted in 5 % dextrose over one hour, whereas severe acidemia may necessitate 1 - 2 mEq/kg over several hours with ventilatory support. In pediatric patients, 1 - 3 mEq/kg infused over 4 - 8 hours is customary, but neonates should receive 4.2 % solutions at rates no greater than 8 mEq/kg/day to avoid intracranial hemorrhage. During cardiac arrest, guidelines permit an initial 1 mEq/kg IV bolus followed by 0.5 mEq/kg every 10 minutes if acidosis persists. Continuous infusions require dedicated lines and infusion pumps to prevent inadvertent rapid bolus[9].
At physiologic pH, sodium bicarbonate dissociates completely to yield sodium cations and bicarbonate anions. The bicarbonate component acts as a weak base that buffers excess hydrogen ions according to the Henderson-Hasselbalch equilibrium, generating carbonic acid that is subsequently converted to carbon dioxide and water by carbonic anhydrase; the carbon dioxide is eliminated via pulmonary ventilation. Restoration of serum bicarbonate concentration increases blood pH and, by mass action, drives hydrogen ions out of cells, indirectly lowering intracellular potassium. The rise in strong ion difference also improves myocardial contractility, but at the expense of a leftward shift of the hemoglobin-oxygen dissociation curve, which can impair tissue oxygen unloading in shock states[3].
Because sodium bicarbonate supplies a hypertonic sodium load, it expands extracellular volume and raises plasma osmolality. Rapid administration may therefore precipitate acute hypernatremia and osmotic shifts that transiently reduce cerebrospinal fluid pressure, a concern particularly in neonates and patients with traumatic brain injury. In addition, bicarbonate-induced alkalemia alters calcium binding to albumin, leading to a decrease in ionized calcium that can provoke neuromuscular irritability or arrhythmias. The drug’s effects are thus tightly linked to renal and respiratory compensation; without adequate ventilation to excrete the generated CO₂ or renal capacity to eliminate excess sodium, paradoxical intracellular acidosis and fluid overload could ensue[4].
Sodium Bicarbonate Injection is contraindicated in patients with pre-existing metabolic or respiratory alkalosis, uncontrolled congestive heart failure, severe renal insufficiency characterized by oliguria or anuria, hypernatremia, or hypocalcemia refractory to correction. Because the solution’s high sodium content can exacerbate fluid retention and systemic hypertension, clinicians should avoid its use in conditions associated with sodium or water overload such as cirrhosis with ascites or generalized edema. Concomitant hypokalemia may be intensified by the intracellular shift of potassium during alkalinization, increasing the risk of ventricular arrhythmias in digitalized patients. Pulmonary edema, especially in neonates and the elderly, has been reported after rapid bolus dosing. Finally, extravasation of the hypertonic solution into subcutaneous tissues is absolutely contraindicated, as it can cause severe chemical cellulitis, ulceration, or necrosis[5].
Sodium bicarbonate is chemically incompatible with most catecholamines, calcium salts, and many antibiotic solutions; co-administration with norepinephrine, dobutamine, or calcium gluconate in the same intravenous line produces precipitation or inactivation. Alkalinization of urine and systemic pH may alter the distribution and elimination of weak acid and base drugs: serum concentrations of salicylates, barbiturates, methotrexate, and certain tetracyclines can decrease as urinary excretion accelerates, whereas drugs such as amphetamines could demonstrate prolonged effects in an alkaline milieu. Co-administration with carbonic anhydrase inhibitors or excessive intake of antacids raises the likelihood of systemic alkalosis. Clinicians should also consider that bicarbonate therapy can blunt the efficacy of vasopressors by increasing serum pH above the optimal range for receptor responsiveness[6].
Common adverse reactions include transient gastric bloating and belching when inadvertent enteral exposure occurs, mild paresthesias during infusion, and increased thirst related to osmotic shifts. More serious events encompass hypernatremia, hypokalemia, metabolic alkalosis with tetany, cerebral hemorrhage in neonates, pulmonary edema, and paradoxical hypercapnia in poorly ventilated patients. Local pain, phlebitis, or tissue necrosis may arise if peripheral veins are used for administration. Rare idiosyncratic phenomena such as Munchausen syndrome with deliberate ingestion, or scalp-vein calcification in premature infants receiving repeated bicarbonate and calcium injections, have been described. Adverse hemodynamic responses, including decreased systemic vascular resistance and hypotension, are reported when large boluses are given during cardiac resuscitation[7].
The use of intravenous sodium bicarbonate during pregnancy is categorized as risk class C because controlled human studies are lacking and animal data are inadequate. Limited clinical observations suggest that maternal alkalinization can transiently increase fetal and umbilical arterial pH, yet the associated sodium load predisposes to edema, weight gain, and potential hypertensive complications. Alternative buffering agents or careful titration of bicarbonate should therefore be considered, reserving therapy for situations where severe, life-threatening acidemia outweighs theoretical risks.
Placental transfer of bicarbonate ions occurs, and neonatal metabolic alkalosis has been documented after vigorous maternal dosing. No data address excretion in breast milk, but given endogenous production of bicarbonate, clinically significant exposure to the nursing infant is unlikely[8].
Vials should be stored at controlled room temperature between 20 °C and 25 °C (68 °F - 77 °F); brief excursions to 15 - 30 °C are permissible. Protect from freezing and excessive heat, as crystallization or loss of potency may occur outside these limits. Photostability studies indicate no clinically relevant degradation after four weeks at temperatures up to 38 °C, but visible inspection for particulate matter, haze, or discoloration is mandatory before each use. Once the vial is penetrated, discard any unused portion because the formulation is preservative-free. Do not autoclave or re-sterilize, and avoid admixture with calcium-containing solutions to forestall precipitation[10].
- DrugBank. (2025). Sodium bicarbonate. DrugBank Online. https://go.drugbank.com/drugs/DB01390
- Pfizer Medical Information. (n.d.). Sodium bicarbonate injection, USP: Warnings and precautions. https://www.pfizermedicalinformation.com/sodium-bicarbonate/warnings
- Drugs..com. (2025). Sodium bicarbonate: Disease interactions. https://www.drugs.com/disease-interactions/sodium-bicarbonate.html
- Yagi, K., & Fujii, T. (2021). Management of acute metabolic acidosis in the ICU: Sodium bicarbonate and renal replacement therapy. Critical Care, 25, 314. https://doi.org/10.1186/s13054-021-03677-4
- American Society of Health-System Pharmacists. (2021). Sodium bicarbonate. In Injectable Drug Information. https://publications.ashp.org/abstract/book/9781585286850/ch351.xml
- Hack, B. (2010). Stability of sodium bicarbonate solutions in polyolefin bags. American Journal of Health-System Pharmacy, 67(12), 1026-1030. https://doi.org/10.1093/ajhp/67.12.1026
- Drugs..com. (2024). Sodium bicarbonate dosage guide. https://www.drugs.com/dosage/sodium-bicarbonate.html
- Jung, B. (2023). Sodium bicarbonate for the treatment of severe metabolic acidosis with acute kidney injury. BMJ Open, 13(8), e073487. https://doi.org/10.1136/bmjopen-2023-073487
- Drugs..com. (2023). Sodium bicarbonate side effects. https://www.drugs.com/sfx/sodium-bicarbonate-side-effects.html
- Drugs..com. (2024). Sodium bicarbonate: Pregnancy and breastfeeding warnings. https://www.drugs.com/pregnancy/sodium-bicarbonate.html
- Pfizer Medical Information. (n.d.). Sodium bicarbonate injection, USP: Adverse reactions. https://www.pfizermedicalinformation.com/sodium-bicarbonate/adverse-reactions
- Siekmann, T., & Kopec, K. (2019). ToxCard: Sodium bicarbonate for toxicology-When to use it and why. emDocs. https://www.emdocs.net/toxcard-sodium-bicarbonate-for-toxicology-when-to-use-it-and-why/
- Critical Care Reviews. (2024). Sodium bicarbonate therapy overview. https://criticalcarereviews.com/therapies/sodium-bicarbonate
- Fresenius Kabi. (2019). Sodium bicarbonate injection, USP: Product monograph. https://editor.fresenius-kabi.us/PIs/US-PH-Sodium_Bicarbonate_Inj-FK-451554A_Sept_2020-PI.pdf
- King Edward Memorial Hospital & Perth Children’s Hospital. (2019). Sodium bicarbonate: Neonatal guidelines. https://www.kemh.health.wa.gov.au/~/media/HSPs/NMHS/Hospitals/WNHS/Documents/Clinical-guidelines/Neonatal-MPs/Sodium-Bicarbonate.pdf
- DailyMed. (2020). Sodium bicarbonate injection, solution. https://fda.report/DailyMed/a26d709b-b294-4b02-9a88-7e840f099a33
- Al-Shaqsi, S. (2016). Burn-like skin necrosis after bicarbonate infusion: A case report. Journal of Clinical Medicine Research, 8(9), 675-678. https://www.jscimedcentral.com/public/assets/articles/article-pdf-1630402238-2080.pdf
- St George’s University Hospitals NHS Foundation Trust. (2008). Critical care intravenous drug administration guide. https://www.gicu.sgul.ac.uk/teaching/resources/pharmacology-and-toxicology/files/itu_IV_guide_-_2008_update_v2.pdf
- Welsh Paediatric Nephrology Network. (2022). Hypernatremia management guidelines. https://cavuhb.nhs.wales/files/welsh-clinical-network-for-paediatric-nephrology/hypernatremia-pdf/
- Pharmaceutical Specialist. (2021). IV drug compatibility chart. https://pharmaceutical-specialist.weebly.com/uploads/7/1/7/3/7173517/ivdrugchart_compatibility.pdf
Q: Is Sodium Bicarbonate Injection the same as baking soda from the grocery store?
No. Pharmaceutical-grade sodium bicarbonate is sterile, pyrogen-tested, and specifically buffered for IV use, whereas household baking soda is not intended or safe for injection[11]
Q: How quickly will the pH rise after an IV bolus?
Partial correction is often seen within minutes as bicarbonate combines with hydrogen ions and CO₂ is exhaled, but full equilibration depends on ventilation and perfusion status[12]
Q: Can I push the drug through a peripheral line in an emergency?
Central access is preferred; if peripheral administration is unavoidable, dilute to 4.2 % and infuse slowly to minimize vein irritation and extravasation injury[13]
Q: What monitoring is needed during therapy?
Serial arterial or venous blood gases, electrolytes, serum osmolality, and fluid balance should be checked every 15-30 minutes initially, then at longer intervals once stable[14]
Q: Does bicarbonate therapy improve survival in lactic acidosis?
Evidence is inconclusive; most guidelines reserve its use for pH ≤ 7.0 with hemodynamic compromise, emphasizing source control and perfusion optimization[15]
Q: Why is bicarbonate incompatible with norepinephrine?
High pH causes catecholamine degradation and precipitation, reducing drug potency and risking line occlusion[16]
Q: What happens if the solution extravasates?
Immediate limb elevation, warm compresses, and local hyaluronidase may limit chemical cellulitis, but severe cases can progress to ulceration or necrosis[17]
Q: Is it useful for tricyclic antidepressant overdose?
Yes; bolus doses can narrow QRS prolongation by alkalinizing serum and providing a sodium load that antagonizes sodium-channel blockade[18]
Q: Can bicarbonate correct hyperkalemia alone?
It offers only temporary intracellular shifting of potassium and should be combined with insulin-glucose, beta-agonists, or dialysis in severe cases[19]
Q: How long can a prepared infusion bag of bicarbonate remain stable?
Studies show chemical stability for at least 24 hours at room temperature in polyolefin containers, but strict aseptic technique dictates discarding after one day[20]
Disclaimer: This compounded medication is prepared under section 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Magnesium Chloride Injection
Magnesium Chloride Injection Calcium Gluconate Injection
Calcium Gluconate Injection Glycine Injection
Glycine Injection Taurine Injection
Taurine Injection Glutathione Injection
Glutathione Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Carnitine (L) Injection
Carnitine (L) Injection Alpha-Lipoic Acid Injection
Alpha-Lipoic Acid Injection BCAA Injection
BCAA Injection