Product Overview
Pyridoxine HCl Injection is a sterile injectable form of vitamin B₆ (pyridoxine) prepared at a concentration of 100 mg/mL. It is typically supplied in multi-dose vials (e.g. 30 mL) and is intended for intramuscular or intravenous administration by healthcare professionals. This formulation is used to treat or prevent vitamin B₆ deficiency and related conditions.
Clinical indications include deficiency due to inadequate diet or malabsorption, drug-induced deficiency (for example, from long-term isoniazid or oral contraceptives), and certain inborn errors of metabolism (such as pyridoxine-dependent seizures or sideroblastic anemia). In infants, injectable pyridoxine is used in the management of some types of neonatal seizures that respond to vitamin B₆ therapy.[1]
Oral pyridoxine supplements are widely available over the counter, but the injectable form is reserved for situations where high doses are needed rapidly, or the oral route is not feasible. This product is compounded and provided through a registered 503B outsourcing facility under U.S. law.[2]
As a 503B-compounded medication, it is not an FDA-approved commercially marketed drug; however, it is prepared in accordance with current good manufacturing practices and quality standards applicable to outsourcing facilities. This allows hospitals and clinics to obtain pyridoxine HCl injections for office use, to ensure patients have access to necessary sterile vitamin B₆ therapy in appropriate clinical situations.[2]
The dosage of Pyridoxine HCl Injection depends on the indication and severity of the vitamin B₆ deficiency or condition being treated. In adults with dietary pyridoxine deficiency (often manifesting as anemia, dermatitis, or neuropathy), a typical regimen is 10 mg to 20 mg of pyridoxine HCl given intramuscularly or intravenously once daily for approximately 3 weeks. This initial course replenishes body stores.
After the acute repletion phase, therapy is usually transitioned to an oral maintenance multivitamin containing a physiological dose of pyridoxine (2-5 mg daily) for several weeks, along with dietary counseling to prevent recurrence. In drug-induced pyridoxine deficiency, such as in patients taking isoniazid, a higher replacement dose is often used, for example, 100 mg IV or IM daily for 3 weeks, to rapidly correct the deficiency, followed by a maintenance dose of around 30 mg daily.
Some inherited metabolic disorders (pyridoxine-dependent conditions) may require very large daily doses; for instance, certain vitamin B₆-dependent epilepsies necessitate doses up to 100-600 mg per day to control seizures, with long-term supplementation of about 30 mg daily for life to prevent symptom recurrence.[13] In the setting of acute isoniazid overdose or toxicity, extraordinarily high doses of pyridoxine are used as an antidote: the recommended approach is to administer pyridoxine in a total amount equal to the amount of isoniazid ingested.
In practice, this involves giving an initial 4 g IV infusion of pyridoxine, followed by repeated 1 g IM injections every 30 minutes until the total matched dose is reached.[13] Such dosing is intended to reverse life-threatening seizures and metabolic effects by restoring adequate coenzyme levels.
Pyridoxine HCl Injection can be given via intramuscular or slow intravenous injection; the route is chosen based on urgency and clinical context. Parenteral administration should always follow proper aseptic technique, and the solution should be inspected for particulates or discoloration before use. The dosage and duration of therapy should be individualized by the physician according to the patient’s response and underlying cause of deficiency.
Pyridoxine is one of three natural forms of vitamin B₆ (along with pyridoxal and pyridoxamine) and is a water-soluble vitamin that must be obtained from diet or supplements. After administration, pyridoxine is converted in the body to its active metabolite, pyridoxal 5′-phosphate (PLP), by the enzyme pyridoxal kinase. PLP serves as a coenzyme in over one hndred enzymatic reactions, playing a crucial role in amino acid metabolism, neurotransmitter synthesis, gluconeogenesis, and lipid and carbohydrate metabolism.[3]
In essence, pyridoxine’s mechanism of action is to restore or maintain levels of PLP, thereby supporting the enzymatic processes that depend on this cofactor.[3] Deficiency of vitamin B₆ leads to impaired function of PLP-dependent enzymes, which can manifest as neurological, hematological, and dermatological symptoms. By providing exogenous pyridoxine that is readily converted to PLP, the injection corrects the coenzyme deficiency and reactivates these metabolic pathways.
For example, PLP is required for the synthesis of neurotransmitters such as GABA and serotonin, for heme biosynthesis (through δ-amino levulinate synthase in red blood cell production), and for amino acid transformations. In conditions like pyridoxine-dependent epilepsy or isoniazid toxicity, pyridoxine administration replenishes PLP stores, thereby restoring normal neurotransmitter levels and terminating seizures.[4]
Thus, the therapeutic effect of pyridoxine HCl Injection arises from normalization of vitamin B₆ coenzyme activity in tissues, enabling proper neuronal function, red blood cell formation, and metabolic homeostasis.
Pyridoxine HCl Injection is contraindicated in patients with a known hypersensitivity to pyridoxine or any component of the formulation. Individuals who have experienced an allergic reaction (e.g. rash, urticaria, or anaphylaxis) to vitamin B₆ or to the excipients in the injection should not receive this therapy.[5]
Apart from true vitamin B₆ allergy (which is rare), there are no absolute contraindications in the general population, because pyridoxine is an essential nutrient. However, the use of excessive dosages in patients with no deficiency is discouraged. Patients who already have exceptionally high pyridoxine levels or signs of pyridoxine toxicity (such as sensory neuropathy) should not continue to receive high-dose supplementation until those symptoms resolve.
Special caution is warranted in certain contexts even if not formal contraindications. For example, injectable pyridoxine solutions may contain aluminum as a trace impurity, and with prolonged use in patients with renal impairment (especially premature neonates with immature kidneys) there is a risk of aluminum accumulation and toxicity. Therefore, in patients with significantly impaired renal function or in infants, the therapy should be used only if clearly indicated and with careful monitoring.
It is also advised to avoid mega doses of pyridoxine in pregnant or breastfeeding patients unless necessary, since safety data for doses beyond the recommended dietary allowance are limited.[6] In summary, aside from hypersensitivity, the use of pyridoxine should be guided by clinical necessity, and caution exercised in populations vulnerable to potential risks of excipients or overdose.
Vitamin B₆ (pyridoxine) can interact with various medications, and these interactions should be considered when administering pyridoxine HCl Injection. One well-documented interaction is with certain anti-epileptic drugs: high-dose pyridoxine may reduce the plasma levels of phenytoin and phenobarbital by enhancing their hepatic metabolism, potentially diminishing their anticonvulsant efficacy.
Concurrent administration of pyridoxine with these medications has been reported to lower their therapeutic levels, so monitoring and dosage adjustments of the anticonvulsant might be required if high-dose vitamin B₆ is given. Pyridoxine also antagonizes the effects of levodopa when levodopa is used without a dopa-decarboxylase inhibitor (such as carbidopa).
In patients with Parkinson’s disease on levodopa monotherapy, vitamin B₆ supplementation can increase peripheral levodopa metabolism and thus reduce the amount of levodopa reaching the central nervous system, worsening symptom control.[7] (This interaction is not significant when levodopa is combined with carbidopa, as in standard carbidopa/levodopa therapy, because carbidopa prevents peripheral levodopa breakdown.)
Another notable interaction involves the chemotherapeutic agent altretamine: coadministration of pyridoxine can decrease altretamine’s effectiveness and is generally advised against unless specifically directed.[7]
Conversely, certain drugs can affect pyridoxine status. Isoniazid (INH), a tuberculosis medication, and hydralazine, used for hypertension, both form complexes with pyridoxal 5′-phosphate or otherwise interfere with vitamin B₆ metabolism, leading to functional vitamin B₆ deficiency. Patients on prolonged INH therapy often receive supplemental pyridoxine to prevent peripheral neuropathy, an adverse effect related to B₆ deficiency.
Other antibiotics such as cycloserine and penicillamine have similar vitamin B₆-antagonizing effects, necessitating supplementation to avert neurological side effects⁸. It is also known that oral contraceptives may lower pyridoxine levels in some individuals, which could be considered a drug-nutrient interaction of clinical relevance.[5]
In summary, pyridoxine may decrease the efficacy of certain medications (notably levodopa and some anticonvulsants), and various drugs can in turn precipitate vitamin B₆ deficiency. Clinicians should review the patient’s medication list and use pyridoxine judiciously, adjusting therapy as needed to account for these interactions.
At physiological or moderately elevated doses, pyridoxine is generally well tolerated, and side effects tend to be mild. Because pyridoxine HCl Injection is a water-soluble vitamin preparation, it does not typically cause systemic toxicity at recommended dosages. Commonly reported minor adverse effects include nausea, headache, somnolence (drowsiness), and mild paresthesia (tingling or numbness) in the extremities.
Some patients receiving intramuscular injections may experience localized injection site reactions such as pain, redness, or irritation, but serious injection site complications are uncommon. Allergic reactions are rare; however, as with any medication, anaphylactic reactions to pyridoxine injection have been reported in isolated cases of hypersensitive individuals. High-dose or prolonged pyridoxine supplementation can also lead to alterations in other nutrient levels, for example, extended use of large doses has been associated with reduced serum folate concentrations in some reports, which suggests a need to monitor nutritional balance during long-term therapy.[9]
The most significant side effect of excessive pyridoxine exposure is peripheral neuropathy affecting sensory nerve function. Chronic high-dose vitamin B₆ intake (generally in gram quantities daily over many months) has been linked to severe sensory neuropathy characterized by numbness, impaired proprioception, and paresthesia in a “stocking-glove” distribution.
Cases of progressive gait instability and loss of balance have been documented in patients taking extremely high doses for extended periods, with symptoms in some instances proving irreversible. Neuropathy is believed to result from pyridoxine neurotoxicity to dorsal root ganglia when plasma levels are excessively high. Notably, these neurotoxic effects are rarely seen at doses below 100 mg/day in adults; typical therapeutic doses are well below the toxic threshold and are considered safe.[10]
Other uncommon adverse effects reported with very high doses include dermatological lesions (photosensitivity and skin rash), gastrointestinal upset, and in some cases dependency syndromes (e.g. a proposed phenomenon where abrupt cessation of 200 mg/day long-term caused withdrawal-like symptoms).[6]
Overall, when used at the prescribed therapeutic doses and duration, side effects of pyridoxine HCl Injection are minimal. Patients are advised to report any unusual numbness, tingling, or coordination problems, as these may signal neurotoxicity if dosing is excessive.[10]
Pyridoxine (vitamin B₆) is generally considered safe for use during pregnancy when administered at recommended doses. Adequate maternal pyridoxine levels are important for fetal development, and the vitamin is present in prenatal vitamins in amounts roughly equivalent to the Recommended Dietary Allowance (around 1.9 mg per day for pregnant women) . There is no evidence of fetal harm from normal physiologic supplementation of vitamin B₆; historically, the FDA categorized pyridoxine as Pregnancy Category A for standard doses, indicating that controlled studies in pregnant women have not shown a risk to the fetus.[11]
Pyridoxine has been extensively used to treat nausea and vomiting of pregnancy, reflecting its safety profile. In clinical practice, the American College of Obstetricians and Gynecologists (ACOG) recommends pyridoxine (10-25 mg three or four times daily) as a first-line treatment for morning sickness, given its efficacy in reducing nausea and its excellent safety record. Pyridoxine’s use in pregnancy, often in combination with doxylamine, has not been associated with teratogenic effects in either the first trimester or later trimesters.[12]
While routine dietary or prenatal vitamin levels of pyridoxine are clearly safe and beneficial in pregnancy, extremely high pharmacological doses are not routinely advised unless there is a clear medical indication.
Doses greatly exceeding the nutritional requirements (for example, hundreds of milligrams daily) have not been studied in randomized trials for safety, so one should avoid mega doses in pregnancy unless treating a specific condition (such as pyridoxine-responsive seizures) under careful supervision.
Notably, older classification systems indicated that using pyridoxine above the usual recommended intake falls under Pregnancy Category C, reflecting that risk cannot be ruled out without more data.[11] In practice, this means that while normal doses are safe, any use of pyridoxine beyond typical supplemental levels during pregnancy should be guided by a healthcare provider’s assessment of risk versus benefit.
Pyridoxine is also considered safe during lactation in normal amounts, although exceedingly high doses might transiently suppress prolactin levels and were historically used (in much larger doses than nutritional needs) to suppress lactation.[11]
Overall, pyridoxine HCl Injection can be used in pregnant patients if needed for deficiency or other indications, with prudent dosing and medical oversight.
Pyridoxine HCl Injection should be stored in accordance with standard recommendations for vitamin solutions to maintain its potency. Vials should be kept at controlled room temperature (generally 20-25 °C), protected from excessive heat and moisture.[14] As the pyridoxine molecule can be gradually degraded by exposure to light, it is advisable to store the vials in their carton or in a light-resistant container to minimize light exposure.
The solution is stable under normal conditions when sealed; however, if the vial is multi-dose and contains a preservative, it should be kept tightly closed between uses to prevent contamination. Do not freeze the vials, as freezing and thawing could potentially precipitate ingredients or compromise the integrity of the container.
Before administration, one should visually inspect the solution: if there are particulate matter, cloudiness, or a change in color, the vial should be discarded in accordance with safe disposal guidelines. Pyridoxine HCl Injection is stable in air but should not be left open to air unnecessarily; always replace the cap or stopper promptly after withdrawing a dose.[1]
Additionally, the product should be stored out of reach of children. By adhering to proper storage conditions, cool, dry, and protected from light, the shelf life of the pyridoxine injection is maximized, and the medication will remain effective until its labeled expiration date.
- Fresenius Kabi USA, LLC. (2024). Pyridoxine Hydrochloride Injection, USP 100 mg/mL [Prescribing information]. DailyMed. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a56d11c0-b033-4201-85ff-fc710506481a
- U.S. Food and Drug Administration. (n.d.). Information for Outsourcing Facilities. Retrieved June 10, 2025, from https://www.fda.gov/drugs/human-drug-compounding/information-outsourcing-facilities
- Abosamak, N. R., & Gupta, V. (2023). Vitamin B6 (Pyridoxine). In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557436/
- DrugBank. (2021). Pyridoxine (DB00165). Retrieved from https://go.drugbank.com/drugs/DB00165
- RxList. (n.d.). Pyridoxine: Side Effects, Uses, Dosage, Interactions, Warnings. Retrieved June 10, 2025, from https://www.rxlist.com/pyridoxine/generic-drug.htm
- Abosamak, N. R., & Gupta, V. (2023). Vitamin B6 (Pyridoxine). In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557436/
- Mayo Clinic. (n.d.). Vitamin B-6 (pyridoxine). Mayo Foundation for Medical Education and Research. https://www.mayoclinic.org/drugs-supplements-vitamin-b6/art-20363468
- Medscape. (n.d.). Pyridoxine (Vitamin B6). WebMD LLC. Retrieved June 10, 2025, from https://reference.medscape.com/drug/vitamin-b6-nestrex-pyridoxine-344425
- Drugs..com. (2025, April 25). Pyridoxine - Uses, Side Effects & Warnings. https://www.drugs.com/mtm/pyridoxine.html
- Therapeutic Goods Administration. (2022, November 10). Health supplements containing vitamin B6 can cause peripheral neuropathy [Safety alert]. Australian Government Department of Health. https://www.tga.gov.au/news/safety-alerts/health-supplements-containing-vitamin-b6-can-cause-peripheral-neuropathy
- Drugs..com. (n.d.). Pyridoxine Use During Pregnancy. Retrieved June 10, 2025, from https://www.drugs.com/pregnancy/pyridoxine.html
- Office of Dietary Supplements. (2022). Vitamin B6 - Fact Sheet for Health Professionals. National Institutes of Health. https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/
- Drugs..com. (2025). Pyridoxine - Uses, Side Effects & Warnings. https://www.drugs.com/mtm/pyridoxine.html
- St. Luke’s Health System. (2017). Pyridoxine (Vitamin B6). Healthwise Knowledgebase. https://www.stlukesonline.org/health-services/health-information/healthwise/2017/06/27/13/09/pyridoxine-vitamin-b6
- Saint Luke’s Health System. (n.d.). Pyridoxine. (Patient education monograph). Retrieved June 10, 2025, from https://www.saintlukeskc.org/health-library/pyridoxine
- Fresenius Kabi USA, LLC. (2024). Pyridoxine Hydrochloride Injection, USP 100 mg/mL [Prescribing information]. DailyMed. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a56d11c0-b033-4201-85ff-fc710506481a
- St. Luke’s Health System. (2017). Pyridoxine (Vitamin B6). Healthwise Knowledgebase. https://www.stlukesonline.org/health-services/health-information/healthwise/2017/06/27/13/09/pyridoxine-vitamin-b6
- Abosamak, N. R., & Gupta, V. (2023). Vitamin B6 (Pyridoxine). In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557436/
- Medscape. (n.d.). Pyridoxine (Vitamin B6). WebMD LLC. Retrieved June 10, 2025, from https://reference.medscape.com/drug/vitamin-b6-nestrex-pyridoxine-344425
- U.S. Food and Drug Administration. (n.d.). Information for Outsourcing Facilities. Retrieved June 10, 2025, from https://www.fda.gov/drugs/human-drug-compounding/information-outsourcing-facilities
- Drugs..com. (2025). Pyridoxine - Uses, Side Effects & Warnings. https://www.drugs.com/mtm/pyridoxine.html
- RxList. (n.d.). Pyridoxine: Side Effects, Uses, Dosage, Interactions, Warnings. Retrieved June 10, 2025, from https://www.rxlist.com/pyridoxine/generic-drug.htm
- Mayo Clinic. (n.d.). Vitamin B-6 (pyridoxine). Mayo Foundation for Medical Education and Research. https://www.mayoclinic.org/drugs-supplements-vitamin-b6/art-20363468
- St. Luke’s Health System. (2017). Pyridoxine (Vitamin B6). Healthwise Knowledgebase. https://www.stlukesonline.org/health-services/health-information/healthwise/2017/06/27/13/09/pyridoxine-vitamin-b6
What is Pyridoxine HCl Injection and what is it used for?
Pyridoxine HCl Injection is an injectable form of vitamin B₆ used to treat or prevent vitamin B₆ deficiency. It is indicated for conditions such as pyridoxine deficiency from poor diet or malabsorption, anemia due to low B₆, and certain cases of neonatal seizures that respond to vitamin B₆ therapy. The injection provides a high-potency dose of pyridoxine for patients who require rapid repletion or cannot take oral vitamin B₆.[15]
Why would someone need an injection of vitamin B₆ instead of an oral supplement?
The injectable form is typically used when oral administration is not feasible or not sufficiently effective. For example, if a patient has a severe deficiency with malabsorption (unable to absorb vitamins from the gut) or is experiencing vomiting that prevents oral intake, an injection ensures the vitamin B₆ is delivered into the body.[16] In urgent situations like isoniazid overdose or vitamin B₆-dependent seizures, only the injection can rapidly achieve the high serum levels of pyridoxine needed to correct the problem.
How is Pyridoxine HCl Injection administered?
This injection is administered by a healthcare professional, either intramuscularly (into a muscle) or intravenously (into a vein). The appropriate route and dose depend on the clinical scenario. Intramuscular (IM) injections are common for routine repletion of B₆ levels, whereas intravenous (IV) administration might be used in emergency situations or when large doses are required quickly. Patients typically receive the injection in a clinic or hospital setting; it is not a self-administered medication.[17]
Are there any side effects associated with Pyridoxine HCl Injection?
Side effects are usually mild at normal doses. Some patients may experience nausea, headache, or a feeling of sleepiness after the injection. At the injection site, there can be temporary pain or redness. Serious adverse effects are very rare. However, if extremely high doses are given for a long time, pyridoxine can cause nerve damage leading to numbness or tingling in the hands and feet (peripheral neuropathy).[18] Any unusual symptoms should be reported to the prescribing healthcare provider.
Can Pyridoxine HCl Injection be used during pregnancy?
Yes, pyridoxine is often used during pregnancy in appropriate doses. It is considered safe and is actually recommended (in oral form) to help treat nausea and vomiting in pregnancy. Standard prenatal vitamins contain vitamin B₆. The injectable form would only be used during pregnancy if there is a clear medical need (such as a severe deficiency or inability to take oral vitamins). High doses beyond the typical daily requirement are avoided unless necessary, but there is no evidence of harm to the fetus from normal vitamin B₆ therapy.[19] Pregnant patients should use this injection only under medical supervision.
Is this product FDA-approved?
Not in the traditional sense. Pyridoxine HCl Injection from a 503B outsourcing facility is a compounded medication and has not undergone the FDA’s pre-market approval process. Under Section 503B of the Federal Food, Drug, and Cosmetic Act, outsourcing facilities can compound sterile drugs like this without individual prescriptions, and these products are exempt from FDA approval requirements (though they must be made under stringent quality standards).[20] In short, the active ingredient (vitamin B₆) is well-known, but the specific compounded formulation is not an FDA-approved branded product.
Do I need a prescription for Pyridoxine HCl Injection?
Yes. Injectable pyridoxine is not available over the counter and must be used under medical supervision. Typically, a licensed healthcare provider orders this medication for a patient. In practice, the injection is administered in a clinical setting by a nurse or physician because it must be given via IV or IM route and monitored.[21] Oral vitamin B₆ supplements can be bought without a prescription, but the injectable form is regulated and dispensed as a prescription product.
Who should not receive Pyridoxine HCl Injection?
Anyone with a known allergy to pyridoxine or any ingredient in the formulation should not receive this injection.[5][22] Signs of an allergic reaction could include rash, itching, swelling, or difficulty breathing.
Aside from allergies, there are no other absolute contraindications to vitamin B₆ in appropriate doses. However, if someone already has extremely high levels of vitamin B₆ (from taking excessive supplements and showing toxicity symptoms), further pyridoxine would be avoided.
It’s also recommended to be cautious in people on certain medications like levodopa (without carbidopa), as mentioned, or those with specific medical conditions, but these are matters of caution rather than strict contraindications.
Can Pyridoxine HCl Injection interact with other medications?
Yes, it can. High-dose pyridoxine may reduce the effectiveness of some medications. For instance, large amounts of vitamin B₆ can increase the metabolism of phenytoin or phenobarbital, potentially lowering their blood levels and reducing seizure control. It can also interfere with levodopa therapy if levodopa is taken without carbidopa, as vitamin B₆ can diminish levodopa’s therapeutic effect.[23] On the other hand, certain drugs like isoniazid, hydralazine, and some penicillamine can cause vitamin B₆ deficiency and might necessitate concurrent pyridoxine supplementation. It’s important to inform the healthcare provider of all the medications being taken so that they can manage any potential interactions.
How should I store Pyridoxine HCl Injection if I have a vial at home or in the clinic?
This injection should be stored at room temperature, away from excessive heat and moisture.[24] Keep the vial in a dry place and do not refrigerate or freeze it. It’s also advisable to protect it from light (for example, keep it in its box) because light can slowly degrade vitamin B₆ over time.[1] Always check the expiration date and do not use the vial beyond that date. If the solution appears cloudy or contains particles, dispose of it as directed and obtain a new vial. As with all medications, store it securely out of reach of children.
Disclaimer: This compounded medication is prepared under section 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
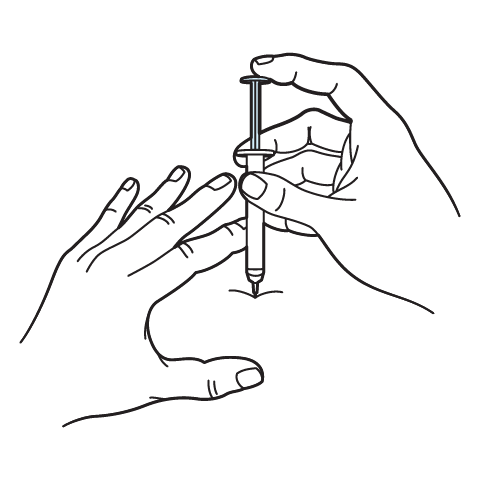
Intramuscular Injection Instructions
Related medications
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Vitamin B-Complex Injection
Vitamin B-Complex Injection Thiamine HCl Injection
Thiamine HCl Injection Folic Acid (Vitamin B9) Injection
Folic Acid (Vitamin B9) Injection Dexpanthenol (Vitamin B5) Injection
Dexpanthenol (Vitamin B5) Injection Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Magnesium Chloride Injection
Magnesium Chloride Injection Calcium Gluconate Injection
Calcium Gluconate Injection Methylcobalamin Injection (Vitamin B12)
Methylcobalamin Injection (Vitamin B12)