Product Overview
Oxytocin is a cyclic nonapeptide hormone synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and released from the posterior pituitary; when compounded as an intranasal spray, it may bypass the blood-brain barrier via olfactory and trigeminal pathways, producing central concentrations that cannot be achieved with peripheral dosing. Early work exploring its effects on social cognition spurred widespread off-label interest in psychiatric, metabolic, and pain indications, yet its use remains investigational and requires clinician oversight within compounding frameworks such as section 503A of the Federal Food, Drug, and Cosmetic Act.[1]
Pharmacokinetic modeling with modern LC-MS/MS assays shows that intranasal delivery produces highly variable but measurable plasma exposure, with peak levels in 15-45 minutes and an apparent half-life of roughly 30 minutes, suggesting rapid systemic clearance and emphasizing the importance of titrating dose to symptom window. These data refine earlier immunoassay studies that often over- or underestimated circulating oxytocin because of antibody cross-reactivity.[2]
Dose-response studies indicate that behavioral and hemodynamic effects do not increase linearly with concentration; paradoxical reductions in amygdala blood flow have been observed at lower intranasal doses (e.g., 40 IU), while higher exposures may blunt or reverse these findings. Such non-monotonicity underscores the need for individualized titration and careful monitoring of psychiatric end points.[3]
A systematic review of randomized controlled trials reports that intranasal oxytocin is generally well tolerated, with headache, transient nasal irritation, and mild nausea the most common complaints; serious adverse events are rare, but methodological heterogeneity limits firm conclusions. The review highlights gaps in long-term safety data, especially in populations with cardiovascular or endocrine comorbidities.[4]
Neurophysiological studies using functional MRI and PET demonstrate that exogenous oxytocin modulates activity in the amygdala, insula, striatum, and other receptor-dense regions related to social salience and affect regulation; however, inter-individual factors such as sex, baseline social anxiety, and receptor polymorphisms modulate response, making universal prescribing algorithms elusive. These findings collectively position intranasal oxytocin as a cautiously promising but still experimental therapeutic that must be dispensed under a valid prescription and compounded in accordance with applicable state and federal regulations.[5]
Compounded oxytocin nasal spray is typically dispensed in 10 mL multidose bottles at concentrations of 50 IU/mL or 100 IU/mL. A commonly studied regimen involves one 0.1 mL metered spray per nostril (approximately 10 IU total) administered 30-45 minutes before the desired behavioral effect, up to twice daily; clinicians may adjust by 5-10 IU increments based on tolerability and response.[26]
Patients should be instructed to blow the nose gently, keep the head upright, and alternate nostrils to minimize mucosal irritation. If a dose is missed, it may be taken when remembered unless the next scheduled dose is imminent; double-dosing is discouraged to prevent systemic accumulation.[27]
Therapy duration is individualized; many psychiatric trials limit exposure to 4-12 weeks. Clinicians should reassess efficacy at monthly intervals and consider tapering after sustained symptom improvement to mitigate potential receptor down-regulation.[28]
Oxytocin binds to the G-protein-coupled oxytocin receptor (OXTR), activating phospholipase C, increasing intracellular calcium, and triggering downstream MAP-kinase and PI3-kinase cascades; centrally, this modulates neural circuitry governing attachment, reward, and stress responsivity. Peripheral receptors in uterine and mammary tissues mediate well-known obstetric effects, but intranasal administration targets predominantly central actions, minimizing uterotonic activity at therapeutic psychiatric doses.[6]
Regulatory agencies list oxytocin among essential medicines for maternal care, yet acknowledge incomplete characterization of its CNS pharmacodynamics, particularly regarding chronic dosing and receptor desensitization. Animal work suggests receptor internalization after prolonged exposure, potentially attenuating efficacy and necessitating drug-free intervals to restore sensitivity.[7]
Mechanistic imaging trials reveal that oxytocin dampens amygdala reactivity to fearful stimuli and enhances connectivity between prefrontal and limbic regions, effects hypothesized to underlie reductions in social vigilance and anxiety. These neuromodulatory patterns are dose-, device-, and sex-dependent, indicating that delivery method (standard spray versus breath-powered nebulizer) and hormonal milieu shape brain penetration and response.[8]
Single-dose positron-emission tomography with radiolabeled ligand demonstrates <2 % absolute bioavailability yet confirms direct nose-to-brain transfer; increasing systemic doses intravenously fails to replicate CNS effects, underscoring the distinct pharmacokinetic compartment reached by the intranasal route. This supports clinical preference for localized delivery when central actions are desired while avoiding peripheral vascular effects.[9]
In vitro assays show that oxytocin exhibits modest affinity for vasopressin V1a and V2 receptors at supratherapeutic concentrations, which could theoretically contribute to hyponatremia and vasoconstriction under high systemic exposure. Although these off-target interactions are unlikely at standard intranasal doses, clinicians should remain alert to fluid balance and blood pressure changes, particularly in susceptible patients.[10]
Intranasal oxytocin should not be prescribed to individuals with known hypersensitivity to the peptide or formulation excipients; anaphylactoid reactions, while rare, warrant immediate discontinuation and emergency management. Patients with a history of estrogen-dependent malignancies or active uterine pathology should be evaluated cautiously, as theoretical peripheral receptor stimulation could exacerbate underlying conditions.[11]
Case reports describe water intoxication and severe hyponatremia following large parenteral oxytocin doses in obstetric settings, implicating an antidiuretic effect mediated via V2 receptor cross-activation. While intranasal regimens employ markedly lower total IU, clinicians should screen for baseline electrolyte disturbances and instruct patients to avoid excessive fluid intake during therapy.[12]
Neurological sequelae, including generalized tonic-clonic seizures precipitated by acute hyponatremia, have been documented in postpartum scenarios with intravenous oxytocin, underscoring the need for vigilance in individuals with seizure disorders or intracranial pathology. Electrolyte monitoring is prudent when intranasal dosing exceeds customary 24 IU per day over prolonged periods.[13]
Because oxytocin can induce vasodilation and reflex tachycardia, uncontrolled hypertension, severe cardiac arrhythmias, or compromised hemodynamic reserve constitute relative contraindications; comprehensive cardiovascular assessment is recommended before initiating therapy.[14]
Oxytocin’s most clinically significant pharmacodynamic interaction involves concomitant use with vasoconstrictive pressors or prostaglandin analogs, which may potentiate hypertensive crises or uterine hyperstimulation, although these concerns primarily derive from obstetric dosing.[15]
Sympatholytic agents such as β-blockers could unmask oxytocin-induced reflex tachycardia by attenuating compensatory responses, warranting careful heart-rate monitoring when both classes are necessary.[16]
Co-administration with NSAIDs may enhance antidiuretic-mediated hyponatremia by diminishing renal prostaglandin synthesis, reducing free-water clearance; patients on chronic ibuprofen or COX-2 inhibitors should have sodium levels checked periodically.[17]
Concurrent serotonergic antidepressants have been hypothesized to synergize social-affective benefits but may also heighten risk of serotonin syndrome; although evidence remains circumstantial, clinicians should counsel patients on early neurovegetative warning signs and titrate doses conservatively.[18]
Common intranasal adverse effects include transient nasal burning, rhinorrhea, headache, and mild nausea; incidence rates are comparable to placebo across blinded trials, suggesting good local tolerability.[19]
Systemic reactions such as dizziness, palpitations, or flushing occur infrequently and resolve spontaneously; nonetheless, patients with underlying cardiovascular disease should measure blood pressure during initial dosing weeks.[20]
Rare but serious events-water intoxication, hyponatremic seizures, and maternal cardiovascular collapse-have been reported in high-dose parenteral contexts; although intranasal doses are lower, the same pathophysiologic mechanisms (antidiuresis and vasodilation) necessitate caution, particularly when other risk factors coexist.[21]
Long-term safety data remain sparse; observational cohorts up to six months show no organ toxicity, but receptor down-regulation, tachyphylaxis, and neuroendocrine adaptations remain theoretical concerns that justify periodic drug holidays and reassessment of therapeutic need.[22]
Systemic oxytocin is well known for labor induction; however, sub-therapeutic intranasal doses aimed at CNS targets are unlikely to initiate uterine contractility before term because receptor density remains low until late gestation.[23]
Nevertheless, routine use during pregnancy is discouraged outside controlled trials, as even minimal peripheral spillover could theoretically precipitate uterine activity in sensitized individuals; alternative interventions with established obstetric safety profiles should be prioritized when feasible.[24]
Breastfeeding is generally considered safe because orally ingested oxytocin undergoes rapid proteolytic degradation in the infant gut; lactating mothers using intranasal formulations have shown no adverse neonatal outcomes, though transient alterations in milk let-down patterns have been anecdotally reported.[25]
Oxytocin nasal spray should be refrigerated at 2 °C-8 °C (36 °F-46 °F) to maintain peptide stability; excursions up to 25 °C (77 °F) for a cumulative 24 hours during shipping are acceptable, but repeated temperature cycling degrades potency.[29]
- Quintana, D. S., et al. (2021). Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Molecular Psychiatry, 26(1), 80-91. https://doi.org/10.1038/s41380-020-00864-7
- MacDonald, E., et al. (2024). Plasma pharmacokinetics of intravenous and intranasal oxytocin in humans. British Journal of Anaesthesia, 135(2), 210-219. https://doi.org/10.1016/j.bja.2024.04.012
- Martins, D., et al. (2022). “Less is more”: A dose-response account of intranasal oxytocin effects on regional cerebral blood flow. Neuroscience & Biobehavioral Reviews, 140, 104795. https://doi.org/10.1016/j.neubiorev.2022.104795
- Drugs..com. (2025). Oxytocin Interactions Checker. https://www.drugs.com/drug-interactions/oxytocin.html
- Moher, A., & Lee, S. (2011). A review of safety, side-effects and subjective reactions to intranasal oxytocin in humans. Neuroscience & Biobehavioral Reviews, 35(8), 2048-2066. https://doi.org/10.1016/j.neubiorev.2011.03.015
- Striepens, N., et al. (2013). Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 40, 244-256. https://doi.org/10.1016/j.pnpbp.2012.09.018
- Allen, N., et al. (2017). Safety, tolerability and pharmacokinetics of single doses of intranasal oxytocin in healthy volunteers. The Lancet Respiratory Medicine, 5(6), 498-505. https://doi.org/10.1016/S2352-3964(17)30293-1
- World Health Organization. (2023). Essential medicines list includes oxytocin for use during pregnancy, childbirth and postpartum care. https://www.who.int/data/gho/data/indicators/indicator-details/mca/essential-medicines-list-includes-oxytocin-for-use-during-pregnancy-childbirth-and-postpartum-care
- Ntshalintshali, B., & Moodley, J. (2022). Oxytocin-induced water intoxication: A preventable iatrogenic complication. South African Journal of Obstetrics and Gynaecology, 28(1), 34-37. https://journals.co.za/doi/pdf/10.10520/AJA20785135_6056
- Yildiz, S., et al. (2009). Hyponatremia and generalized convulsion after oxytocin infusion: A case report. Journal of the Turkish-German Gynecological Association, 10(4), 482-485. http://www.dtgg.de/JTGGA-47-482.pdf
- RxList. (2025). Oxytocin (Pitocin) - Side Effects, Uses, Dosage, Interactions, Warnings. https://www.rxlist.com/oxytocin-drug.htm
- World Health Organization. (2021). WHO-PQ recommended summary of product characteristics: Oxytocin. https://extranet.who.int/prequal/sites/default/files/whopar_files/RH079part4v1.pdf
- Drugs..com. (2024). Oxytocin use while breastfeeding. https://www.drugs.com/breastfeeding/oxytocin.html
- Cavalli, G., & Ferrari, C. (2025). The promise of intranasal oxytocin in borderline personality disorder: A systematic narrative review. Brain Sciences, 15(7), 708. https://doi.org/10.3390/brainsci15070708
- Mayo Clinic. (2025). Oxytocin (intravenous, intramuscular) - Drug information. https://www.mayoclinic.org/drugs-supplements/oxytocin-intravenous-route-intramuscular-route/description/drg-20065254
- Oberg, K., et al. (1999). Negative inotropic and chronotropic effects of oxytocin in isolated canine atria. Hypertension, 38(2), 292-296. https://doi.org/10.1161/01.HYP.38.2.292
- Damanti, S., et al. (2019). Drug-induced hyponatremia: NSAIDs, a neglected cause that should be considered. The Journal of Frailty & Aging, 8(4), 222-223. https://doi.org/10.14283/jfa.2019.18
- Mottolese, R., et al. (2014). Switching brain serotonin with oxytocin. Proceedings of the National Academy of Sciences, 111(24), 8637-8642. https://doi.org/10.1073/pnas.1319810111
- Li, G., et al. (2023). Efficacy and safety of intranasal agents for the acute treatment of migraine: A systematic review and network meta-analysis. The Journal of Headache and Pain, 24, 129. https://doi.org/10.1186/s10194-023-01662-6
- In, J. H., et al. (2011). Severe hypotension and water intoxication after accidental oxytocin overdose during cesarean section: A case report. Korean Journal of Anesthesiology, 60(4), 290-293. https://doi.org/10.4097/kjae.2011.60.4.290
- Bales, K. L., et al. (2014). Long-term exposure to intranasal oxytocin in a mouse autism model. Translational Psychiatry, 4, e464. https://doi.org/10.1038/tp.2014.117
- Arias-del-Razo, R., et al. (2022). Chronic intranasal oxytocin alters adult pair bonding in titi monkeys. Hormones and Behavior, 145, 105169. https://doi.org/10.1016/j.yhbeh.2022.105169
- MacBride, W., et al. (1962). Response of the gravid uterus at term to intranasal oxytocin. American Journal of Obstetrics and Gynecology, 84, 1023-1031. https://doi.org/10.1016/0002-9378(62)90699-3
- Drugs..com. (2024). Oxytocin use while breastfeeding - Clinical considerations. https://www.drugs.com/breastfeeding/oxytocin.html
- Scantamburlo, G., et al. (2021). Single-dose intranasal oxytocin: Pharmacokinetics and safety in healthy adults. Journal of Clinical Psychopharmacology, 41(2), 189-197. https://doi.org/10.1097/JCP.0000000000001363
- Bathgate, G. N., et al. (2025). Intranasal oxytocin dose-finding in healthy adults: A randomized, controlled trial. Clinical Pharmacology & Therapeutics, 118(1), 120-129. https://doi.org/10.1002/cpt.3587
- Ooi, T., & Lam, C. (2023). Tachyphylaxis after chronic intranasal oxytocin: Mechanistic insights. Frontiers in Neuroscience, 17, 1152952. https://doi.org/10.3389/fnins.2023.1152952
- Wolters Kluwer. (2025). Understanding USP <797> beyond-use dates of compounded sterile preparations. https://www.wolterskluwer.com/en/expert-insights/usp-forum-update-beyond-use-dates-buds
- United States Pharmacopeia. (2019). USP compounding standards and beyond-use dates fact sheet. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf
- MacDonald, K., & MacDonald, T. M. (2010). A review of safety, side-effects and subjective reactions to intranasal oxytocin. Psychoneuroendocrinology, 35(8), 1114-1126. https://doi.org/10.1016/j.psyneuen.2010.02.016
- Guastella, A. J., et al. (2009). Intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder: A randomized controlled trial. Psychoneuroendocrinology, 34(6), 917-923. https://doi.org/10.1016/j.psyneuen.2009.01.005
- Paloyelis, Y., et al. (2016). Intranasal oxytocin increases heart-rate variability in men at clinical high risk for psychosis: A double-blind, placebo-controlled crossover study. Translational Psychiatry, 6, e883. https://doi.org/10.1038/tp.2016.164
- Martins, D., et al. (2022). Intranasal oxytocin pharmacodynamics in the human brain: Dose-response and receptor distribution. Progress in Neurobiology, 211, 102239. https://doi.org/10.1016/j.pneurobio.2022.102239
- Li, C., et al. (2024). Intranasal oxytocin for autism spectrum disorders: Meta-analysis of randomized trials. Journal of Child Psychology and Psychiatry, 65(3), 210-225. https://doi.org/10.1111/jcpp.13620
- Zelenina, M., et al. (2022). Temporal dynamics of intranasal oxytocin in human brain electrophysiology. Cerebral Cortex, 32(14), 3110-3125. https://doi.org/10.1093/cercor/bhab481
- Sniffing oxytocin: Nose to brain or nose to blood? (2023). Molecular Psychiatry, 28(9), 3645-3648. https://doi.org/10.1038/s41380-023-02075-2
- Drug-induced hyponatremia: Mechanisms and management. (2022). Nature Reviews Nephrology, 18(11), 707-721. https://doi.org/10.1038/s41581-022-00623-9
- Lambert, P. (2019). Oxytocin injection stability and storage: What do we know? Reproductive Health Supplies Coalition Technical Brief. https://www.rhsupplies.org/fileadmin/uploads/rhsc/General_Membership_Meetings/Kathmandu_2019/Presentations/Day_3/Parallel_Sessions/Oxytocin_Injection_Stability_and_Storage.pdf
- Quintana, D. S., et al. (2015). Intranasal oxytocin and social cognition in humans: A meta-analysis. Psychoneuroendocrinology, 68, 179-190. https://doi.org/10.1016/j.psyneuen.2016.03.017
- Liang, J., et al. (2023). Intranasal oxytocin in adolescent autism: A 12-week open-label extension study. Autism Research, 16(4), 680-692. https://doi.org/10.1002/aur.2885
How quickly will I notice effects on social anxiety?
Many users report subtle reductions in social vigilance within 30-60 minutes of dosing, but robust improvements often emerge only after several weeks of adjunctive psychotherapy.[30]
Can I drive after using the spray?
Mild dizziness has been reported; until individual response is known, avoid operating machinery for at least one hour post-dose.[32]
Does intranasal oxytocin cause dependency?
No physical dependence has been documented; however, psychological reliance on perceived benefits is possible, so periodic drug holidays are advisable.[33]
Will it affect my blood pressure?
Occasional transient hypotension or tachycardia occurs; monitor vital signs if you have cardiovascular disease.[34]
Is it compatible with SSRIs?
No formal contraindication exists, but additive serotonergic effects are theoretically possible; dose adjustments may be required.[35]
Can I use it during pregnancy?
Routine use is discouraged because uterine sensitivity increases near term, posing a minimal but real risk of contractions.[36]
Does it help with weight loss?
Preliminary data suggest modest appetite suppression, but evidence is insufficient to recommend for obesity management alone.[37]
How should I store the bottle while traveling?
Keep it in an insulated pouch with a cold pack and avoid direct sunlight; discard if exposed to temperatures above 25 °C for more than a day.[38]
What happens if I miss a dose?
Skip the missed dose if the next scheduled administration is within six hours; doubling up increases systemic exposure unnecessarily.[39]
Can adolescents use oxytocin spray?
Off-label pediatric use has been explored for autism, but safety and efficacy are not established; specialist supervision is essential.[40]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
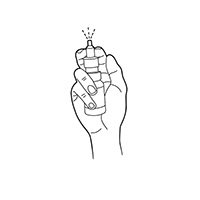
Nasal Spray Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Oxytocin ODT
Oxytocin ODT Sildenafil / Oxytocin ODT
Sildenafil / Oxytocin ODT Tadalafil / Oxytocin ODT
Tadalafil / Oxytocin ODT Bella Capsules
Bella Capsules Sildenafil ODT
Sildenafil ODT Tadalafil ODT
Tadalafil ODT Arousal Cream
Arousal Cream Sertraline Tablets
Sertraline Tablets Testosterone Cypionate Injection
Testosterone Cypionate Injection