Product Overview
Glutathione Injection is a compounded prescription medication formulated for intravenous or intramuscular administration, designed to deliver the antioxidant glutathione directly into the bloodstream. It is available in both preserved and preservative-free 30 mL vials, each containing 200 mg/mL of the active ingredient, glutathione.[1][3]
This sterile injectable solution may be intended for use in patients requiring enhanced antioxidant support, detoxification, or adjunctive therapy in specific medical conditions. As a compounded medication, Glutathione Injection is not commercially manufactured or FDA-approved for any specific indication, and its use should be guided by a qualified healthcare professional.[1][3]
The unique aspect of this formulation lies in its high concentration and the option for preservative-free preparation, which may be suitable for patients with sensitivities or allergies to common preservatives. Glutathione, the sole active ingredient, is a tripeptide composed of glutamine, cysteine, and glycine, and is recognized for its critical role in cellular defense against oxidative stress, as well as its involvement in numerous metabolic and detoxification pathways.[1][3]
The compounded nature of this product allows for individualized dosing and administration, which may be advantageous in clinical scenarios where standardized commercial products are not available or appropriate.[1][3]
Glutathione Injection is typically administered intravenously or intramuscularly by a healthcare professional. The specific dosage and frequency are determined by the prescribing provider based on the patient’s clinical condition, therapeutic goals, and response to treatment.[1][6]
Common dosing protocols in clinical studies have ranged from 600 mg to 1,400 mg per session, administered two to three times per week, though higher or lower doses may be used depending on individual needs. The injection should be prepared and administered using aseptic technique to minimize the risk of infection or contamination.[1][6]
As a compounded product, the dosing regimen may be tailored to each patient’s requirements, and adjustments may be made based on clinical response and tolerability. The duration of therapy varies depending on the indication and patient response, with some protocols recommending treatment for several weeks to months.[1][6]
Glutathione Injection exerts its effects primarily through the pharmacological actions of glutathione, a naturally occurring intracellular antioxidant. Glutathione functions as a key regulator of redox homeostasis, directly neutralizing reactive oxygen species and free radicals, thereby protecting cells from oxidative damage. It also serves as a substrate for various antioxidant enzymes, including glutathione peroxidase and glutathione reductase, which further amplify its protective effects.[4][5]
When administered intravenously or intramuscularly, exogenous glutathione is rapidly distributed in the plasma, where it may be oxidized or broken down into its constituent amino acids. These amino acids can then be taken up by cells and resynthesized into glutathione intracellularly. Notably, intravenous glutathione has a short plasma half-life, with most of the administered dose being cleared from circulation within minutes. Despite this, transient increases in plasma glutathione and its metabolites may support antioxidant defenses, modulate immune responses, and influence cellular signaling pathways. Some studies suggest that glutathione may inhibit melanogenesis by interfering with the activity of tyrosinase, an enzyme critical for melanin synthesis, which has led to its use in skin-lightening protocols; however, the clinical significance and durability of these effects remain under investigation.[6][7]
Glutathione Injection should not be used in individuals with a known hypersensitivity or allergy to glutathione or any component of the formulation. Patients with a history of allergic reactions to similar compounds or excipients present in the preserved formulation should exercise caution.[1][4]
The safety of glutathione injection in patients with severe hepatic or renal impairment has not been established, and its use in these populations should be carefully considered and monitored by a healthcare provider. Additionally, individuals with asthma should avoid inhaled forms of glutathione, as it may exacerbate respiratory symptoms; while this risk is less relevant for injectable forms, caution is still advised in patients with reactive airway diseases.[1][4]
There is insufficient evidence regarding the safety of glutathione injection in pregnancy and lactation, and its use in these populations should be avoided unless the potential benefits outweigh the risks. As with any compounded medication, administration should be performed by qualified personnel familiar with sterile injection techniques and patient monitoring.[1][4]
Glutathione may interact with certain medications and therapies. Notably, it can potentially affect the efficacy or side-effect profile of drugs such as nitroglycerin, nitrates, and some chemotherapy agents. Caution is advised when glutathione is administered concurrently with these medications, as it may alter their metabolism or pharmacodynamics.[2][5]
High doses of acetaminophen are known to deplete endogenous glutathione stores, particularly in the liver, which may increase the risk of toxicity; thus, patients using acetaminophen chronically or in large amounts should be monitored closely when receiving glutathione supplementation.[2][5]
There are no well-documented interactions with most other commonly prescribed medications, but individual responses may vary. Patients should inform their healthcare provider of all medications and supplements they are taking to minimize the risk of adverse interactions, and healthcare professionals should evaluate each regimen prior to initiating glutathione therapy.[2][5]
The administration of Glutathione Injection may be associated with both common and rare side effects. Common adverse reactions include local injection-site discomfort, redness, or swelling. Systemic side effects may include nausea, gastrointestinal upset, flushing, and, in rare cases, allergic reactions such as rash, hives, or anaphylaxis.[5][7]
Some patients may experience increased flatulence or loose stools. There have been isolated reports of reversible hepatic injury following intravenous glutathione administration, emphasizing the need for careful monitoring, especially with repeated or high-dose therapy.[5][7]
At dosages exceeding 5 grams, there is a potential risk for more severe adverse effects, including renal dysfunction, blood toxicity, and neurological symptoms. Overall, glutathione is considered to have a favorable safety profile when used as directed, but the long-term safety of high-dose or chronic use has not been fully established.[5][7]
There is insufficient data regarding the safety of Glutathione Injection during pregnancy and lactation. Animal studies are limited and human data are lacking, making it difficult to assess potential risks to the fetus or nursing infant.[1][4]
As a precaution, glutathione injection should be avoided during pregnancy unless the potential benefits clearly outweigh the risks and only under the supervision of a qualified healthcare provider. Similarly, the excretion of glutathione into breast milk and its effects on the nursing infant are not well understood; therefore, use during breastfeeding should be approached with caution.[1][4]
Healthcare providers should consider alternative therapies with established safety profiles in pregnant or lactating patients whenever possible, and if glutathione injection is deemed necessary, close monitoring of both mother and child is recommended throughout treatment.[1][4]
Proper storage of glutathione injection is important to maintain its stability and potency. Upon receiving the medication, it should be kept in a refrigerator at 36 °F to 46 °F (2 °C to 8 °C) and protected from light. Do not allow the vials to freeze; if a vial does accidentally freeze, it should be discarded. The medication can be kept at room temperature for short periods during preparation, but prolonged exposure should be avoided.[1]
Store the vials in their original carton or in an opaque container within the refrigerator to minimize light exposure. Keep all medications out of reach of children and pets, preferably in a designated medication area. Before each use, inspect the vial; the solution should be clear and colorless (or slightly pale yellow). If you notice particles, cloudiness, or discoloration, do not use that vial and contact the pharmacy for guidance.[2]
After puncturing the rubber stopper for the first dose, the vial becomes a multi-dose container. Use a new sterile needle and syringe for each withdrawal to maintain sterility. Discard any unused portion 28 days after first puncture or by the beyond-use date indicated on the label, whichever comes first. Mark the date of first use on the vial as a reminder and never stretch the use of a vial beyond the advised period.[3]
When disposing of unused medication or expired vials, do not flush them down the toilet or pour them into drains. Instead, follow local guidelines for medication disposal-many pharmacies have take-back programs or specific instructions for safe disposal of injectables. Used needles and syringes should be placed in an FDA-cleared puncture-proof sharps container rather than household trash to prevent needle-stick injuries.[4]
- WebMD. (n.d.). Glutathione - Uses, Side Effects, and More. Retrieved May 6, 2025, from https://www.webmd.com/vitamins/ai/ingredientmono-717/glutathione
- MedIndia. (2024, November 8). Glutathione - Indications, Dosage, Side Effects and Precautions. Retrieved May 6, 2025, from https://www.medindia.net/doctors/drug_information/glutathione.htm
- Drugs..com (2024, July 15). Glutathione Uses, Benefits & Dosage. Retrieved May 6, 2025, from https://www.drugs.com/npp/glutathione.html
- Biom Probiotics. (2023, November 22). Avoiding Risks: Glutathione Supplementation Tips. Retrieved May 6, 2025, from https://biomprobiotics.com/what-to-avoid-when-taking-glutathione-a-guide-to-safe-supplementation/
- SkinKraft. (2022, August 10). Benefits & Side Effects of Glutathione Injections. Retrieved May 6, 2025, from https://skinkraft.com/blogs/articles/glutathione-benefits-and-side-effects
- Ross Bridge Medical Center. (2022, June 1). How Do Glutathione Injections Work? Retrieved May 6, 2025, from https://www.rossbridgemedicalcenter.com/how-do-glutathione-injections-work/
- Burick Center. (2023, April 19). The Benefits of Glutathione Injections and Where to Get Them. Retrieved May 6, 2025, from https://burickcenter.com/the-benefits-of-glutathione-injections-and-where-to-get-them/
- National Institutes of Health, Office of Dietary Supplements. (n.d.). Glutathione. Retrieved May 6, 2025, from https://ods.od.nih.gov/factsheets/Glutathione-HealthProfessional/
What is Glutathione Injection used for?
Glutathione Injection is used as an antioxidant therapy, for detoxification support, and as an adjunct in certain medical conditions where enhanced antioxidant capacity is desired. It is also sometimes used off-label for skin lightening, though evidence for this indication is limited.[1][3]
How is Glutathione Injection administered?
It is administered intravenously or intramuscularly by a healthcare professional, with dosing tailored to the individual patient’s needs and clinical response.[4][5]
Is Glutathione Injection safe?
When used as directed and under medical supervision, glutathione injection is generally well tolerated. However, side effects can occur, and long-term safety data are limited. Patients should be monitored for adverse reactions.[6]
Can Glutathione Injection be used during pregnancy?
There is insufficient evidence regarding the safety of glutathione injection during pregnancy or breastfeeding. Its use should be avoided unless specifically recommended by a healthcare provider after a careful risk-benefit assessment.[7]
How should Glutathione Injection be stored?
Store at controlled room temperature or as directed by the compounding pharmacy. Protect from light and moisture, and do not use beyond the expiration date.[8]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.[1]
Administration Instructions
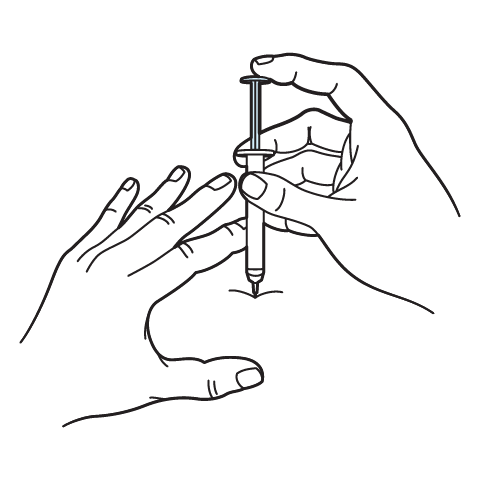
Intramuscular Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.



 Alpha-Lipoic Acid Injection
Alpha-Lipoic Acid Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Arginine HCl Injection
Arginine HCl Injection Glycine Injection
Glycine Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection BCAA Injection
BCAA Injection Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection Pyridoxine HCl Injection
Pyridoxine HCl Injection