Product Overview
Arousal Cream is a pharmacist-compounded, non-sterile topical preparation that combines six vasoactive and neuromodulatory agents-aminophylline, ergoloid mesylate, pentoxifylline, sildenafil citrate, testosterone, and L-arginine-into a single emollient base intended for localized dermal application to external genital skin.
By integrating multiple mechanisms that facilitate smooth-muscle relaxation, microvascular recruitment, and sensory neural modulation, the formulation is designed to assist clinicians in managing female sexual arousal disorder (FSAD) and other conditions characterized by insufficient genital blood flow or diminished arousal response.[1]
The cream is produced under section 503A of the Federal Food, Drug, and Cosmetic Act, meaning it is compounded pursuant to a valid patient-specific prescription, in a state-licensed pharmacy, and is not subject to FDA approval of safety or efficacy.
Its individualized nature allows prescribers to tailor active-ingredient profiles and concentrations to patient-specific therapeutic goals while avoiding excipients that may provoke irritation.
Because compounded preparations lack large-scale clinical trials, prescribers must rely on available ingredient-specific data, published case series, and pharmacologic rationale when selecting this therapy, and patients should receive counseling that outcomes vary and that systemic absorption, while typically low, can occur.[2]
Apply a pea-sized amount (≈0.5 mL) to the external genitalia (clitoral hood, labia minora, and vestibular mucosa) 15-30 minutes before sexual activity, not exceeding two applications in 24 hours.[25]
For maintenance protocols aimed at enhancing baseline responsiveness, some clinicians prescribe nightly application for eight weeks before reassessment.
Individualized titration considers sensitivity to vasoactive effects: patients reporting facial flushing, headache, or palpitations should reduce frequency or quantity.
Dosage variations among formulations 1-5 permit androgen-free options for individuals with contraindications to testosterone or hormonally sensitive conditions.
Pediatric usage, rectal, or intravaginal administration is not recommended.
Therapy is adjunctive to psychosexual counseling and should be discontinued if meaningful improvement is not observed within three months.[26]
The formulation’s therapeutic concept is synergistic vasodilation. Sildenafil citrate inhibits phosphodiesterase-5, preserving cyclic GMP and promoting nitric-oxide-mediated cavernous smooth-muscle relaxation; topical delivery allows high local concentrations with minimal systemic exposure.[3]
L-Arginine acts as a substrate for nitric-oxide synthase, potentially amplifying endothelial nitric-oxide production and supporting clitoral and vestibular engorgement.[4]
Testosterone may potentiate sexual motivation centrally and augment peripheral nitric-oxide pathways; transdermal absorption produces targeted androgenic signaling while avoiding hepatic first-pass metabolism.[5]
Aminophylline, a non-selective phosphodiesterase inhibitor, increases intracellular cAMP and could enhance lipolysis in subdermal adipose, theoretically reducing tissue turgor and improving drug penetration.[6]
Pentoxifylline heightens microcirculatory flow by reducing blood viscosity and enhancing erythrocyte deformability, which may facilitate oxygenation of genital tissues and accelerate drug diffusion.[7]
Ergoloid mesylate, an ergot alkaloid mixture, exerts alpha-adrenergic modulation and cerebral vasodilatory effects that may indirectly influence sexual arousal through improved cognitive processing of stimuli.[8]
Together, these agents address both vascular and neuroendocrine contributors to FSAD, offering a multifaceted strategy when first-line monotherapies prove inadequate.[9]
Use is contraindicated in individuals with known hypersensitivity to any component; in patients concurrently receiving nitrates or potent nitric-oxide donors because sildenafil-induced systemic vasodilation could precipitate severe hypotension; in those with active migraine or Raynaud phenomenon where ergoloid mesylate may exacerbate vasospasm; in severe hepatic impairment where testosterone metabolism is unpredictable; and in persons with uncontrolled bleeding disorders, peptic-ulcer disease, or history of intracerebral hemorrhage, given pentoxifylline’s antiplatelet effects and potential testosterone-related alterations in clotting factors.[10][11]
Patients with arrhythmias, uncontrolled hypertension, or recent myocardial infarction should avoid aminophylline-containing products due to positive chronotropic effects, and those with hormone-sensitive malignancies (e.g., breast, endometrial) must not use testosterone-containing variants. Because safety data in pediatrics are absent, Arousal Cream is restricted to adult use.
Concomitant application on broken or inflamed skin is contraindicated owing to increased systemic uptake risk.[12]
Significant pharmacokinetic and pharmacodynamic interactions stem from the diverse ingredient profile. Co-administration of organic nitrates or riociguat with sildenafil can trigger profound hypotension; alpha-blockers may synergistically lower systemic blood pressure.[13]
Caffeine and other xanthines potentiate aminophylline’s central nervous system stimulation, raising arrhythmia risk, while beta-blockers may exhibit diminished efficacy when high dermal aminophylline concentrations are absorbed.[14]
Concurrent use of strong CYP3A4 inhibitors (e.g., ketoconazole, ritonavir) may elevate sildenafil and testosterone levels, necessitating interval spacing or dose reduction.
Warfarin coupled with testosterone has been linked to increased international normalized ratio (INR) and bleeding episodes, warranting enhanced monitoring.[15]
Pentoxifylline combined with anticoagulants or antiplatelet agents (aspirin, clopidogrel, direct oral anticoagulants) can augment hemorrhagic risk through additive platelet inhibition.[16]
Ergoloid mesylate may interact with serotonergic agents, heightening serotonin syndrome potential, and with potent vasoconstrictors, increasing ischemic complications.
Because topical absorption is variable, clinicians should still assess systemic medication lists as though oral dosages were used.[17]
Localized adverse reactions-transient erythema, warmth, pruritus, or mild burning-occur in up to one-third of users and are generally self-limiting; occlusive dressings or application to recently shaved skin can intensify irritation.[18]
Systemic events are uncommon but possible: mild headache, nasal congestion, flushing, palpitations (sildenafil-related), tachycardia or insomnia (aminophylline), lightheadedness or dizziness (pentoxifylline), androgenic effects such as acne, fine hair growth, or mood changes (testosterone), and rare paresthesias (ergoloid mesylate).
Excessive application or impaired skin barrier can increase bioavailability, potentiating hypotension, priapism, or arrhythmogenic events, necessitating prompt medical evaluation.
Allergic contact dermatitis, though rare, has been documented with compounded bases and should prompt discontinuation and allergen testing.
Long-term safety beyond six months of continuous daily use has not been established; periodic reassessment is advised.[19]
Each active ingredient carries distinct reproductive-risk profiles. Sildenafil’s limited human data suggest acceptable maternal tolerance, but fetal benefit remains unproven, and systemic vasodilation could compromise placental perfusion in susceptible patients.[20]
Pentoxifylline and aminophylline are pregnancy category C; animal studies show embryotoxicity at supra-therapeutic doses, and human case reports describe placental passage, so use is justified only when potential benefit outweighs risk.[21]
Testosterone-containing variants are strictly contraindicated in pregnancy (category X) because virilization of a female fetus can occur with even minimal systemic absorption.[22]
L-Arginine supplementation has been investigated for preeclampsia prevention and intrauterine growth restriction; although generally considered safe, topical pharmacokinetics during gestation are undefined.[23]
Because inadvertent dermal transfer to partners or neonates is possible, pregnant individuals or those attempting conception should avoid exposure to the cream, and caregivers should employ barrier methods or washable gloves during application.[24]
Store tightly closed at 20 °C to 25 °C (68 °F - 77 °F). Protect from excessive moisture, heat, and direct sunlight to maintain chemical stability and emulsion integrity.
- Urology Times. (2024). Topical sildenafil cream found safe and tolerable in female sexual arousal disorder. https://www.urologytimes.com/view/topical-sildenafil-cream-found-safe-tolerable-in-female-sexual-arousal-disorder
- Department of Veterans Affairs. (2025, March). Transdermal testosterone (off-label) for hypoactive sexual desire disorder: Clinical summary. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Testosterone_HSDD_Clinical_Summary_Mar_2025.pdf
- Berkeley Life Professional. (2024). Nitric oxide: A critical key to female sexual wellness. https://berkeleylifeprofessional.com/wp-content/uploads/2023/08/WP_Female-Sexual-Wellness-NO_2.pdf
- Esmaeili, S. M., & colleagues. (2023). The efficacy of topical aminophylline in local fat reduction: A systematic review. Frontiers in Endocrinology, 14, Article 1087614. https://doi.org/10.3389/fendo.2023.1087614
- Deng, G., & colleagues. (2023). Efficacy and safety of pentoxifylline for chronic venous leg ulcers: A systematic review and meta-analysis. Trials, 24, 547. https://doi.org/10.1186/s13063-023-07547-y
- Drugs..com. (2024). Ergoloid mesylates: Professional monograph. https://www.drugs.com/monograph/ergoloid-mesylates.html
- GoodRx. (2024). Sildenafil interactions to be aware of. https://www.goodrx.com/sildenafil/interactions
- Drugs..com. (2024). Aminophylline: Professional patient advice. https://www.drugs.com/ppa/aminophylline.html
- Ferreira, R., & colleagues. (2017). Sildenafil in pregnancy: A systematic review of maternal tolerance and obstetric outcomes. Fetal Diagnosis and Therapy, 41(2), 81-89. https://doi.org/10.1159/000447745
- United States Pharmacopeia. (2023). USP compounding standards and beyond-use dates fact sheet. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf
- Trials Journal. (2023). Pentoxifylline for venous leg ulcers meta-analysis. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-023-07547-y
- Drugs..com. (2024). Aminophylline interactions checker. https://www.drugs.com/drug-interactions/aminophylline.html
- .UK Clinical Pharmacy Association. (2024). Pentoxifylline-Perioperative medicines handbook. https://periop-handbook.ukclinicalpharmacy.org/drug/pentoxifylline/
- Drugs..com. (2024). Testosterone and warfarin interaction. https://www.drugs.com/drug-interactions/testosterone-with-warfarin-2167-0-2311-0.html
- Drugs..com. (2024). Pentoxifylline use during pregnancy. https://www.drugs.com/pregnancy/pentoxifylline.html
- Drugs..com. (2024). Aminophylline use during pregnancy. https://www.drugs.com/pregnancy/aminophylline.html
- Centers for Disease Control and Prevention. (2024). Testosterone use and risk for pregnancy. https://www.cdc.gov/contraception/hcp/usspr/testosterone-pregnancy-risk.html
- Ferri, N., & colleagues. (2023). L-Arginine supplementation in pregnancy: A systematic review. Journal of Maternal-Fetal & Neonatal Medicine. https://doi.org/10.1080/14767058.2023.2217465
- Pourcelot, A., & colleagues. (2019). The effects of sildenafil on maternal and fetal outcomes in pregnancy: A systematic review. PLOS ONE, 14(7), e0219732. https://doi.org/10.1371/journal.pone.0219732
- FDA. (2024). TRENTAL (pentoxifylline) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018631s039lbl.pdf
- American Journal of Obstetrics and Gynecology. (2022). Prenatal exposure to teratogenic medications in the era of REMS. https://doi.org/10.1016/j.ajog.2022.01.008
- Food and Drug Administration. (2023). Product-specific guidances for topical products. https://www.fda.gov/media/173387/download
- Burnett, A. L. (2009). Endothelial nitric oxide synthase regulation in female genital tract and sexual arousal. Journal of Sexual Medicine, 6(Suppl 3), 247-253. https://doi.org/10.1111/j.1743-6109.2008.01122.x
- Alkilani, A., & colleagues. (2024). Advancements in transdermal drug delivery: A comprehensive review of penetration-enhancer strategies. International Journal of Pharmaceutics, 636, 122806. https://doi.org/10.1016/j.ijpharm.2024.122806
- Parish, S. J., Simon, J. A., Davis, S. R., Giraldi, A., Goldstein, I., & International Society for the Study of Women’s Sexual Health. (2021). International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. Journal of Sexual Medicine, 18(5), 849-867. https://doi.org/10.1016/j.jsxm.2020.10.009
- Parish, S. J., & Kling, J. M. (2023). Testosterone use for hypoactive sexual desire disorder in postmenopausal women (NAMS Practice Pearl). North American Menopause Society. https://www.huntingtonhealth.org/wp-content/uploads/2024/09/NAMS_practice-pearl-testosterone_.pdf
How quickly does Arousal Cream work after application?
Most patients report perceivable warmth or engorgement within 10-20 minutes as local vasodilation develops.[11]
Can it be used with systemic PDE-5 inhibitors like oral sildenafil?
Combination is generally discouraged because additive hypotension and priapism risk outweigh potential benefit.[12]
Is the product scented or flavored?
The default base is unscented.
Will testosterone in the cream cause virilizing side effects?
At prescribed dermal doses systemic absorption is low, but transient acne or fine facial hair can appear in some users; routine monitoring mitigates concerns.[14]
Can partners absorb the medication transdermally?
Yes; partners should avoid direct contact with treated skin until the area is washed, or the product fully dries to prevent systemic exposure.[15]
What if a dose is accidentally applied to broken skin?
Wash immediately; compromised barrier can accelerate systemic uptake and elevate adverse-event risk.[16]
Does alcohol intake affect efficacy or safety?
Moderate alcohol use may potentiate hypotension from sildenafil; excessive intake increases vasodilatory dizziness and should be avoided.[17]
How long will a 30 mL jar last at recommended dosing?
At 0.5 mL per dose, one jar provides roughly 60 applications-about two months for users applying before each sexual encounter twice weekly.[18]
Can menopausal hormone therapy be continued concurrently?
Yes, but clinicians should review estrogen doses because combined vasodilatory effects may alter blood pressure and vascular reactivity.[19]
Is there a risk of developing tolerance over time?
No pharmacologic tolerance is documented; nevertheless, psychological adaptation may blunt perceived benefit, warranting periodic drug holidays.[20]
How should leftover product be disposed of?
Return unused cream to the pharmacy for chemical disposal; do not flush or discard in household trash to avoid environmental contamination.[21]
Does insurance cover compounded Arousal Cream?
Most insurers classify it as non-formulary; patients typically self-pay, but health-savings accounts may reimburse with a prescription.[23]
Can men use this cream for erectile dysfunction?
Limited data exist; male penile application might improve tumescence, but compounded strengths and hormonal components are optimized for vulvar tissue, so off-label male use is not recommended.[24]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
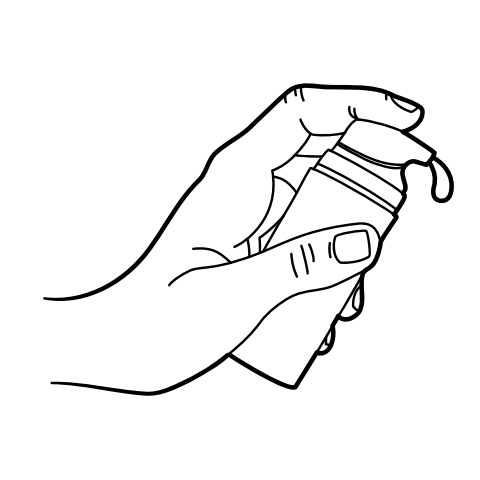
Pump Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.






 Oxytocin ODT
Oxytocin ODT Oxytocin Nasal Spray
Oxytocin Nasal Spray Testosterone Vaginal Cream
Testosterone Vaginal Cream Testosterone Cypionate Injection
Testosterone Cypionate Injection Sertraline Tablets
Sertraline Tablets