Product Overview
Alpha-lipoic acid (ALA, also known as thioctic acid) is an endogenous antioxidant compound involved in mitochondrial energy metabolism. ALA has been utilized for its potential therapeutic effects in conditions associated with oxidative stress. The first clinical use of alpha-lipoic acid was reported in 1959 in Germany, initially for treating Amanita mushroom (death cap) poisoning.[1]
Since then, ALA has been extensively studied in diabetic peripheral neuropathy and diabetes mellitus, among other conditions. Notably, it has been used in Germany as an approved treatment for diabetic neuropathy for decades.[1] In the United States, ALA is available as a dietary supplement and as a compounded injectable formulation (25 mg/mL in a 30 mL vial) prepared by compounding pharmacies and outsourcing facilities.[2]
Research indicates that ALA may improve symptoms of diabetic neuropathy: for example, clinical trials and meta-analyses have shown that intravenous ALA (typically 600 mg per day for 3 weeks) may significantly reduce neuropathic pain, paresthesia, and numbness in diabetic patients.[2][4] However, evidence for ALA’s efficacy in other conditions (such as cognitive disorders, liver disease, or other neuropathies) is limited or inconclusive.[1]
It is important to recognize that Alpha-Lipoic Acid Injection is a compounded prescription medication, not an FDA-approved commercially manufactured drug. Compounded ALA injections are formulated by licensed pharmacies and outsourcing facilities like Empower Pharmacy on a patient-specific and office use basis in accordance with Section 50.
General Dosing Considerations: Alpha-Lipoic Acid Injection is administered parenterally (typically by intravenous infusion), and dosing should be individualized by the prescribing physician according to the patient’s needs and response. There is no single “one-size-fits-all” dosage for ALA, as it may be used for different purposes. However, clinical experience and studies provide guidance on common dosing regimens.
In adults, the most frequently studied dosing range for intravenous alpha-lipoic acid is 300 mg to 600 mg per day.[19] A dose of 600 mg IV daily is often used, as this was the standard in many clinical trials for diabetic neuropathy and has been associated with symptom improvements. For example, controlled trials in diabetic polyneuropathy have employed 600 mg of ALA injected IV once daily (often given as an infusion over 30-60 minutes) for periods of 2-4 weeks, with significant efficacy observed in reducing neuropathic symptoms compared to placebo.[19][20] An initial intensive treatment phase (e.g., daily IV therapy for several weeks) is sometimes followed by an oral maintenance dose in longer-term management, based on German clinical protocols for neuropathy.[20]
It is important to note that doses higher than 600 mg/day intravenously have not been shown to confer additional clinical benefit, but they may increase side effects.[15] Thus, 600 mg/day can be considered a typical maximum IV dose for most indications, unless a provider prescribes otherwise for a specific situation. Some patients and conditions might use lower doses (e.g., 100-300 mg IV) especially if the goal is general antioxidant support or if higher doses are not tolerated, but evidence is strongest for the 600 mg IV regimen in neuropathy. Always, the dosage and frequency should be determined by a qualified healthcare provider familiar with the patient’s health status.
Administration: Alpha-Lipoic Acid Injection must be administered in a clinical setting or under appropriate medical supervision. The IV injection is usually prepared by diluting the required dose of ALA in a compatible solution (such as normal saline) and given by slow intravenous infusion to minimize potential side effects (like dizziness or vein irritation). The infusion typically lasts around 30 minutes (for 600 mg) but can vary. Because ALA is light-sensitive, pharmacies often provide it in light-resistant packaging and it should be administered shortly after preparation to ensure stability. Patients receiving IV alpha-lipoic acid might notice a slight odor (ALA has a sulfur-containing structure) during infusion, this is normal.
Monitoring: During IV therapy, medical staff will monitor blood glucose in diabetic patients (due to the risk of hypoglycemia) and observe for any allergic reactions or infusion-site reactions. Treatment courses can vary in length; for diabetic neuropathy, a common protocol is daily IV infusions for 2-4 weeks as an initial therapy.[19][20] In other cases, clinicians might use intermittent dosing schedules. After an IV course, if continued therapy is needed, patients are often switched to oral ALA supplements (since oral forms are readily available for maintenance therapy).
It is crucial that patients follow the dosage instructions provided by their healthcare provider. Do not adjust the dose or frequency of Alpha-Lipoic Acid Injection on your own. If a dose is missed, consult your provider for guidance; do not double up doses. Given that this medication is compounded specifically for each patient, any dosage changes must be authorized by the prescriber, and a new preparation may be required to reflect a new strength if needed.
Alpha-lipoic acid’s mechanism of action is multifaceted. Chemically, ALA contains a dithiolane ring that can undergo reduction to dihydrolipoic acid (DHLA) in the body. Both ALA and its reduced form DHLA act as potent antioxidants, capable of scavenging reactive oxygen species in various cellular compartments.[5] Uniquely, ALA is often termed a “universal antioxidant” because it is soluble in both water and lipid environments, and it has the ability to regenerate other antioxidants such as vitamin C, vitamin E, and glutathione from their oxidized forms.[5][6]
In addition, ALA chelates redox-active metal ions like iron and copper, which helps prevent metal-catalyzed free radical formation.[5] Beyond its antioxidant properties, ALA may serve as an essential cofactor for mitochondrial enzymatic complexes (notably the α-ketoacid dehydrogenases) involved in oxidative metabolism.[6] This role in energy production may contribute to its therapeutic effects in metabolic conditions.
ALA has also been observed to enhance insulin-stimulated glucose uptake in skeletal muscle, thereby improving insulin sensitivity in patients with type 2 diabetes.[7] These combined actions, antioxidative, metal-chelating, and metabolic, underlie ALA’s potential benefits in mitigating oxidative damage and improving metabolic function.
It is worth noting that ALA can modulate inflammatory pathways as well; for instance, some studies indicate that ALA supplementation can decrease activation of the NF-κB cytokine pathway, suggesting an anti-inflammatory effect (though this is an area of ongoing research).[6]
Alpha-Lipoic Acid Injection is contraindicated in individuals with a known hypersensitivity or allergy to alpha-lipoic acid or any of the formulation’s components. There are no other absolute contraindications definitively established for ALA; however, several conditions warrant caution and careful medical supervision when considering ALA therapy.[8]
Patients with diabetes mellitus should use ALA with caution because ALA can enhance glucose utilization and insulin sensitivity, potentially leading to hypoglycemia (low blood sugar) if used alongside insulin or oral antidiabetic drugs. Those on antidiabetic therapy who begin ALA treatment should monitor their blood glucose closely and may require adjustments in their diabetes medication dosage to avoid hypoglycemic episodes.[8]
Similarly, individuals with thyroid disorders should be aware that ALA might affect thyroid hormone levels; there are reports that ALA can lower circulating thyroid hormone (T3/T4) or reduce the efficacy of thyroid medications like levothyroxine, which means thyroid function should be monitored if ALA is used concurrently.[8]
Patients with significant alcohol intake or thiamine deficiency also require precaution: chronic heavy alcohol use can deplete thiamine (vitamin B₁) and taking ALA in the setting of thiamine deficiency has been associated with serious health risks (including a condition resembling Wernicke’s encephalopathy). Therefore, individuals who consume alcohol heavily should ensure adequate thiamine supplementation before and during ALA therapy.[9]
In general, any patient with nutritional deficiencies, liver disease, or other serious comorbidities should use ALA only under a physician’s guidance, with appropriate monitoring. If any signs of an allergic reaction occur (such as rash, hives, itching, swelling, or difficulty breathing), use of ALA should be discontinued immediately and medical attention sought.
Alpha-lipoic acid may interact with certain medications, and patients should inform their healthcare provider of all drugs and supplements they are taking to avoid adverse interactions. One major interaction concern is with antidiabetic medications (including insulin and oral hypoglycemic agents). ALA can synergistically enhance blood glucose uptake, thereby potentially causing blood sugar to drop more than expected.[10] Patients on medications for diabetes who start ALA therapy should monitor their blood glucose closely and may need dose adjustments of their diabetic medications to prevent hypoglycemia.[10][11]
Another interaction is with thyroid hormones such as levothyroxine. Alpha-lipoic acid appears to decrease the efficacy of thyroid hormone replacement; it has been noted that taking ALA can lower levels of circulating thyroid hormone or interfere with peripheral conversion of T₄ to T₃.[11] As a result, patients on thyroid medication should have their thyroid function monitored if ALA is added, and dosing of thyroid medication may need to be adjusted by their doctor.[10][11]
In addition, due to its antioxidant properties, ALA might interact with certain chemotherapy or radiation therapies. Some anticancer treatments (e.g., alkylating agents and certain chemotherapy drugs) rely on pro-oxidative mechanisms to kill cancer cells. There is a theoretical concern (and some limited clinical evidence) that high-dose antioxidants like ALA could reduce the efficacy of these treatments. Therefore, cancer patients should not take alpha-lipoic acid (or any supplement) without consulting their oncologist; any consideration of ALA use in a patient undergoing chemotherapy/radiation must be under direct medical guidance.[12]
Lastly, ALA may have mild anticoagulant effects (by enhancing blood flow and endothelial function), so combining it with anticoagulant or antiplatelet drugs could very slightly increase bleeding tendency; patients on blood thinners should use caution and discuss with their provider, although significant bleeding issues with ALA are not commonly reported.[11]
In summary, while alpha-lipoic acid is generally compatible with many medications, it can amplify blood sugar-lowering drugs and alter thyroid hormone activity, among other interactions, so proactive monitoring and consultation with healthcare professionals are essential.[10][12]
Tolerance: Alpha-lipoic acid is considered to be relatively well-tolerated in most adults. Clinical studies, including long-term trials (up to four years of oral supplementation), have found that ALA has a strong safety profile with no significant adverse effects at usual doses. In fact, an adult may take doses up to about 2400 mg/day of ALA without severe harmful effects in the short term, although higher doses do not necessarily confer additional benefit.[13]
Common Side Effects: The most commonly reported side effects of ALA are mild gastrointestinal and constitutional symptoms. These include nausea, vomiting, heartburn, and upset stomach, as well as occasional headache.[13][14] Such side effects, when they occur, are usually transient and resolve on their own. A small number of individuals have reported skin reactions (e.g. rash or itching) or other hypersensitivity-type symptoms, but these are uncommon.[14] Because ALA can lower blood sugar, some patients (especially those with diabetes) may experience signs of mild hypoglycemia, such as dizziness, sweating, or lightheadedness, during ALA therapy if their blood sugar drops too low.[15] It is important for patients prone to hypoglycemia to monitor their blood glucose and recognize these symptoms.
Dose-Related Effects: Notably, the incidence of side effects appears to be dose-dependent. At higher dosages of ALA, side effects become more frequent. For example, in oral dosing trials for diabetic neuropathy, patients receiving 1200 mg to 1800 mg per day of ALA had a greater occurrence of nausea, vomiting, and vertigo compared to those receiving 600 mg per day.[14][15] In those studies, the lowest dose (600 mg) tended to be as well-tolerated as placebo, with no significant difference in adverse events compared to placebo.[15] This suggests that 600 mg/day may be an optimal upper-limit dose for most patients in terms of balancing efficacy and tolerability.
Serious Adverse Events: Serious side effects with alpha-lipoic acid are very rare. There have been isolated case reports of severe toxicity, almost exclusively in situations of extreme overdose or accidental ingestion by small children. In several reported pediatric cases, ingestion of large amounts of alpha-lipoic acid (for example, 2400 mg in a toddler) led to serious reactions such as seizures, loss of consciousness, or metabolic acidosis. Although such outcomes are unusual, they underscore that ALA, like any active substance, can be dangerous in massive overdose. Moreover, a few cases of intentional high-dose ALA overdose have resulted in significant harm, including liver failure and, in one adolescent, a fatal outcome.[16]
Therefore, it is crucial to adhere to recommended dosages and to keep alpha-lipoic acid (especially the concentrated injection vials) out of reach of children. Patients are advised to report any unexpected symptoms to their healthcare provider, and clinicians will typically monitor for symptoms of hypoglycemia or gastrointestinal upset during therapy.
The safety of alpha-lipoic acid use during pregnancy has not been firmly established, and ALA should be used in pregnancy only with extreme caution if at all. There is limited human data on ALA in pregnant women. A few small studies and case reports have not flagged major safety concerns; for instance, oral doses up to 600 mg daily have been used for several weeks in pregnant women (such as those with gestational diabetes) without evident harm. These preliminary reports suggest that short-term, moderate-dose ALA supplementation was tolerated in pregnancy and may have some beneficial metabolic effects (e.g., improved blood sugar control or liver enzyme levels in gestational diabetes).[17]
However, no large-scale, controlled trials have evaluated the efficacy or safety of ALA for pregnant patients, and thus there is insufficient evidence to consider it definitively safe. Most experts and guidelines advise against using alpha-lipoic acid during pregnancy due to the lack of robust data.[18] Animal studies of ALA in pregnancy are also limited, and while some have not shown overt teratogenic effects, they are not fully conclusive. Therefore, ALA is usually only considered in pregnancy if the potential benefit clearly outweighs any theoretical risk, and only under close medical supervision.
In practice, women who are pregnant are generally advised to avoid taking ALA beyond the amounts naturally found in food. Likewise, for breastfeeding, there is no reliable information on the excretion of alpha-lipoic acid into breast milk or its effects on a nursing infant.[18] Because of these unknown, breastfeeding mothers are recommended not to use supplemental or high-dose ALA.
In summary, while small observational uses of alpha-lipoic acid in pregnancy have not reported acute problems, the absence of comprehensive safety data means ALA is not routinely recommended for pregnant or lactating women.[17][18] Women of childbearing potential should inform their healthcare provider if they are taking ALA or planning to start it, and pregnant patients should only use alpha-lipoic acid if it is prescribed by a physician who has judged that the expected benefits outweigh the possible risks.
Alpha-Lipoic Acid Injection should be stored properly to maintain its potency. Vials of ALA injection should be kept at controlled room temperature, ideally around 20°-25°C (68°-77°F). It is recommended to store the vial in a dry place protected from light; many ALA preparations are light-sensitive, and the pharmacy will often supply them in amber vials or light-protective packaging. Do not refrigerate or freeze the vial unless instructed otherwise by the pharmacy. Always keep medications out of reach of children and pets (preferably in a locked medicine cabinet or a high shelf) to prevent accidental ingestion or tampering.[21]
Pay attention to the beyond-use date or expiration date on the pharmacy label. Because this is a compounded medication, it may have a shorter shelf life than manufactured pharmaceuticals. Do not use the injection if the beyond-use date has passed or if the solution appears discolored, cloudy, or contains precipitate.
When a vial is partially used or no longer needed, any remaining solution should be disposed of in a safe and responsible manner. Do not flush unused alpha-lipoic acid solution down the toilet or pour it into household drains, as this can contaminate water supplies. Instead, follow FDA guidelines for medication disposal; ideally, take unused medication to a drug take-back program or return it to a pharmacy that offers medication disposal services.[22] If those options are not available, you may dispose of it in the trash by mixing the solution with an unpalatable substance (like used coffee grounds or cat litter) in a sealed bag before discarding, in accordance with FDA recommendations.
- Gomes, M. B., & Negrato, C. A. (2014). Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & Metabolic Syndrome, 6(1), 80. DOI: 10.1186/1758-5996-6-80
- Nguyen, H., Pellegrini, M. V., & Gupta, V. (2024). Alpha-Lipoic Acid. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. (NCBI Bookshelf ID: NBK564301)
- Ziegler, D., Nowak, H., Kempler, P., Vargha, P., & Low, P. A. (2004). Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabetic Medicine, 21(2), 114-121.
- Mijnhout, G. S., Kollen, B. J., Alkhalaf, A., Kleefstra, N., & Bilo, H. J. G. (2012). Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. International Journal of Endocrinology, 2012, Article ID 456279. https://doi.org/10.1155/2012/456279
- Packer, L., Witt, E. H., & Tritschler, H. J. (1995). Alpha-lipoic acid as a biological antioxidant. Free Radical Biology & Medicine, 19(2), 227-250.
- Shay, K. P., Moreau, R. F., Smith, E. J., Smith, A. R., & Hagen, T. M. (2009). Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochimica et Biophysica Acta, 1790(10), 1149-1160.
- Konrad, T., Vicini, P., Kusterer, K., et al. (1999). α-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care, 22(2), 280-287.
- Drugs..com. (n.d.). Alpha-Lipoic Acid - Warnings and Precautions. Retrieved May 25, 2025, from Drugs.com website: https://www.drugs.com/mtm/alpha-lipoic-acid.html
- MedlinePlus. (2021). Alpha-Lipoic Acid - Precautions. National Library of Medicine, NIH. Retrieved from https://medlineplus.gov/druginfo/natural/767.html
- Ehrlich, S. D. (2007). Alpha-Lipoic Acid - Potential Drug Interactions. In A.D.A.M. Medical Encyclopedia (republished by Lima Memorial Health System). Review Date: 6/18/2007.
- WebMD. (n.d.). Alpha-Lipoic Acid - Uses and Risks. Retrieved May 2025, from WebMD website: https://www.webmd.com/vitamins-supplements/ingredientmono-767-alpha-lipoic%20acid
- Memorial Sloan Kettering Cancer Center. (n.d.). Alpha-Lipoic Acid - For Healthcare Professionals (Integrative Medicine). (Updated 2022). Retrieved from https://www.mskcc.org/cancer-care/integrative-medicine/herbs/alpha-lipoic-acid
- MedlinePlus. (2021). Alpha-Lipoic Acid - Side Effects. National Library of Medicine, NIH. Retrieved from https://medlineplus.gov/druginfo/natural/767.html
- WebMD. (n.d.). Alpha-Lipoic Acid - Side Effects. Retrieved May 2025, from WebMD website: https://www.webmd.com/vitamins-supplements/ingredientmono-767-alpha-lipoic%20acid
- Ziegler, D., Ametov, A., Barinov, A., et al. (2006). Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care, 29(11), 2365-2370.
- MedlinePlus. (2021). Alpha-Lipoic Acid - Overdose and Toxicity. National Library of Medicine, NIH. Retrieved from https://medlineplus.gov/druginfo/natural/767.html
- MedlinePlus. (2021). Alpha-Lipoic Acid - Pregnancy and Breastfeeding. National Library of Medicine, NIH. Retrieved from https://medlineplus.gov/druginfo/natural/767.html
- Nguyen, H., Pellegrini, M. V., & Gupta, V. (2024). Alpha-Lipoic Acid - Warnings in Pregnancy. In StatPearls [Internet]. StatPearls Publishing. (Last Update: Jan 26, 2024)
- Han, T., Bai, J., Liu, W., & Hu, Y. (2012). A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. European Journal of Endocrinology, 167(4), 465-471.
- Ametov, A. S., Strokov, I. A., Kartashev, A. V., et al. (2003). The sensory symptoms of diabetic polyneuropathy are improved with α-lipoic acid: the SYDNEY trial. Diabetes Care, 26(3), 770-776.
- TriVue Pharmaceuticals, Inc. (2020). Oveeza - Storage and Handling. Package Insert for Oveeza (folic acid/methylcobalamin/α-lipoic acid, etc.). DailyMed - U.S. National Library of Medicine. [“Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C.”]
- U.S. Food and Drug Administration (FDA). (2020). Disposal of Unused Medicines: What You Should Know. FDA Drug Safety Communication. Retrieved from https://www.fda.gov/drugs/disposal-unused-medicines-what-you-should-know
Administration Instructions
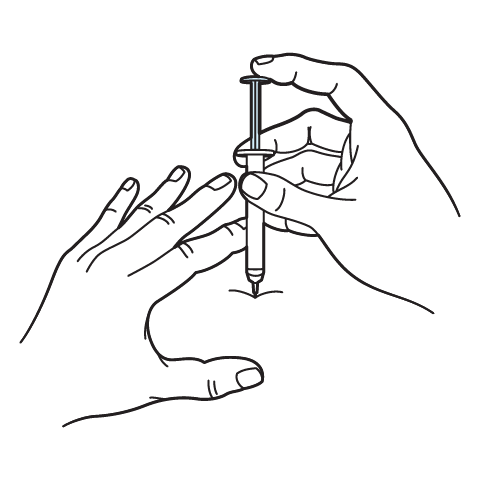
Intramuscular Injection Instructions
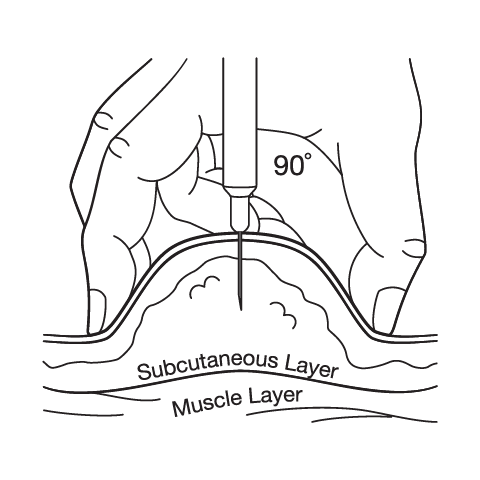
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Arginine HCl Injection
Arginine HCl Injection Glycine Injection
Glycine Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection Pyridoxine HCl Injection
Pyridoxine HCl Injection BCAA Injection
BCAA Injection Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection