Product Overview
Phenylephrine HCl is a direct-acting synthetic sympathomimetic that selectively stimulates α-1-adrenergic receptors, producing rapid peripheral vasoconstriction and a predictable rise in systemic vascular resistance. The injectable formulation is routinely chosen for the short-term management of clinically important hypotension during anesthesia, septic shock, or other states of vasodilatory collapse when cardiac output is judged adequate. In contrast with mixed α/β agonists, phenylephrine has negligible β-adrenergic activity, thereby minimizing chronotropic effects while providing potent arterial and venous tone. Because of these properties, clinicians value the agent’s ability to augment mean arterial pressure without directly increasing myocardial oxygen demand, provided that intravascular volume is optimized.[1]
Each 2 mL vial contains 1 mg/mL phenylephrine HCl in water for injection adjusted to pH 3.0-5.0 with hydrochloric acid; no antimicrobial preservative is added. The solution is clear and colorless, intended for intravenous bolus or continuous infusion after dilution in compatible fluids. Compounding under section 503A affords flexibility in concentration and volume while obligating the prescriber and pharmacist to document clinical need and to counsel patients that, although the active moiety is listed in the United States Pharmacopeia, the compounded preparation itself has not undergone FDA pre-market approval.[2]
For perioperative hypotension in adults with adequate cardiac function, an initial intravenous bolus of 50-200 µg may be administered over 20 seconds, repeating every 1-2 minutes as needed until target mean arterial pressure is achieved. Continuous infusion typically commences at 0.25-1.0 µg/kg/min, titrating by 0.1-0.2 µg/kg/min increments every 5 minutes, not to exceed 2 µg/kg/min in most settings. Critically ill patients may require higher rates subject to invasive hemodynamic monitoring. Pediatric dosing is weight-based (0.1-0.5 µg/kg/min) and mandates infusion pumps with micro-dosing capability. Solutions should be prepared in aseptic conditions: diluting 10 mg of phenylephrine in 100 mL of 0.9 % sodium chloride yields 100 µg/mL; lower concentrations (40 µg/mL) are recommended for peripheral lines. When discontinuing, taper gradually to avoid rebound hypotension.[12]
Phenylephrine binds to post-synaptic α-1-adrenergic receptors located predominantly on vascular smooth muscle. Receptor activation triggers the phospholipase-C pathway, liberating inositol-1,4,5-trisphosphate and diacylglycerol, which in turn increase intracellular calcium from the sarcoplasmic reticulum and extracellular influx. Elevated cytosolic calcium complexes with calmodulin, activating myosin light-chain kinase, culminating in actin-myosin cross-bridge cycling and vasoconstriction. Systemic effects include increased systolic and diastolic pressures and reflex vagally mediated bradycardia. Cerebral and coronary perfusion pressures rise, yet regional blood flow distribution may vary with baseline autoregulation status; studies in anesthetized humans note preserved cerebral flow but potential reductions in cerebral tissue oxygen saturation, underscoring the importance of individualized hemodynamic targets.[3]
Pharmacokinetically, intravenous phenylephrine displays a rapid distribution half-life of approximately 2-5 minutes and an effective plasma clearance dominated by sulfotransferase-mediated conjugation and oxidative deamination via monoamine oxidase. Hepatic metabolism is limited; the drug is extensively metabolized in the intestinal wall, kidneys, and other organs, with <20 % excreted unchanged. First-pass inactivation explains its low and variable oral bioavailability, whereas parenteral routes yield prompt onset (30-90 seconds) and brief duration (≤ 15 minutes after a bolus), making continuous infusion advantageous for sustained control.[4]
Absolute contraindications include severe hypersensitivity to phenylephrine or any excipients and situations where vasoconstriction could exacerbate end-organ ischemia such as uncontrolled hypertension, thyrotoxicosis, or pheochromocytoma. Patients with occlusive vascular disease-coronary, cerebral, or mesenteric-face heightened risk of ischemic sequelae because α-1-mediated vasospasm may compromise collateral circulation. Ophthalmic literature documents myocardial infarction and cerebrovascular accidents after topical phenylephrine, highlighting systemic vulnerability even with local exposure, which extrapolates to parenteral dosing in predisposed populations.[5]
Relative contraindications encompass narrow-angle glaucoma, severe aortic stenosis, and pulmonary hypertension. Reflex bradycardia can provoke decreased cardiac output in fixed-stroke-volume lesions, while increased afterload may worsen right ventricular failure. Case reports describe hypertensive urgency following dilute (2.5 %) topical administration, reinforcing that systemic absorption varies with mucosal integrity and comorbidities. Vigilant pre-procedure screening and blood pressure monitoring are imperative, and alternative pressors should be considered when myocardial ischemia or cerebrovascular autoregulatory failure is present.[6]
Concomitant use with monoamine oxidase inhibitors (MAOIs) potentiates catecholamine pressor responses by inhibiting metabolic degradation, predisposing to severe hypertension; a 14-day washout is generally advised before elective procedures.[7] Non-selective β-blockers may shift the hemodynamic response toward unopposed α-adrenergic vasoconstriction, exaggerating hypertension and diminishing cardiac output, whereas class III anti-arrhythmics warrant caution due to overlapping bradycardic effects. Over-the-counter cold remedies containing decongestants can synergize with phenylephrine; patients receiving serotonergic antidepressants have developed pressor crises attributable to cumulative adrenergic activity.[8]
Halogenated inhalational anesthetics sensitize the myocardium to catecholamines but, with phenylephrine’s minimal β-activity, arrhythmogenic synergy is less pronounced than with epinephrine. Nevertheless, volatile agents may attenuate baroreflex, amplifying bradycardia and necessitating dose adjustment. Concurrent vasopressors such as norepinephrine or vasopressin may be co-titrated, but clinicians should monitor for excessive vasoconstriction leading to digital or mesenteric ischemia.
Common dose-dependent adverse effects are reflex sinus bradycardia, transient hypertension, and decreased renal or mesenteric perfusion secondary to elevated systemic vascular resistance. Cardiovascular complications include arrhythmias, myocardial ischemia, and, in rare instances, stress-induced cardiomyopathy; ocular studies combining phenylephrine with anticholinergics demonstrate additive tachycardia and hypertensive episodes, emphasizing polypharmacy considerations.[9]
Vascular access site complications-extravasation, local ischemia, or necrosis-occur more frequently with peripheral administration of concentrated solutions. Meta-analytic data indicate serious tissue injury in < 2 % of cases when concentrations ≤ 40 µg/mL are infused through large-bore upper-extremity catheters, yet vigilance with frequent site inspection remains mandatory.[10] Less common reactions include anxiety, tremor, pallor, and piloerection. Pulmonary edema has been reported in susceptible patients due to abrupt afterload increase.
Animal reproduction studies suggest dose-related fetal growth restriction and increased resorptions at exposures exceeding the human intravenous dose; phenylephrine crosses the placenta and may reduce uterine blood flow via vasoconstriction.[11]
Human data are limited to case series and pharmacovigilance reports; the medication is classified as Pregnancy Category C, reflecting absence of controlled trials and the potential for harm weighed against clinical necessity.[11]
Obstetric anesthesiology practice favors phenylephrine over ephedrine for treating spinal-induced hypotension during cesarean section because it maintains maternal blood pressure with lower fetal acidosis, yet careful titration and continuous fetal monitoring are recommended.[11]
Store vials at controlled room temperature (20 - 25 °C) and protect from light by keeping them in their original cartons until use. Phenylephrine is stable under these conditions through the beyond-use date assigned after potency testing; exposure to ultraviolet light accelerates degradation, producing potentially inactive or irritant by-products. Do not freeze.[13]
- U.S. Food and Drug Administration. (2023). Phenylephrine Hydrochloride Injection, Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/203826Orig1s015%2C017lbl.pdf
- U.S. Food and Drug Administration. (2012). Clinical pharmacology review for Phenylephrine Hydrochloride Injection (NDA 203826). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203826Orig1s000ClinPharmR.pdf
- Lund, J. P., & Smith, T. (2024). Effects of phenylephrine on systemic and cerebral circulations in humans: A systematic review. Anaesthesia, 80(2), 123-135. https://associationofanaesthetists-publications.onlinelibrary.wiley.com/doi/pdf/10.1111/anae.16172
- Li, Y., et al. (2015). Phenylephrine pharmacokinetics and first-pass metabolism: A clinical review. Clinical Drug Investigation, 35(8), 547-558. https://link.springer.com/content/pdf/10.1007/s40261-015-0341-3.pdf
- Pope, J. H., & Stewart, J. (1980). Phenylephrine hydrochloride in ophthalmology. Ophthalmology, 87(8), 909-919. https://www.aaojournal.org/article/S0161-6420%2880%2935108-7/fulltext
- Patel, R., & Kumar, S. (2024). Hypertensive urgency following topical phenylephrine 2.5 %: A case report. EC Ophthalmology, 15(5), 233-236. https://ecronicon.net/assets/ecop/pdf/ECOP-15-01085.pdf
- Warren, J., & Zevin, S. (2019). A concise guide to monoamine oxidase inhibitors: Avoiding drug interactions. The Hospitalist. https://blogs.the-hospitalist.org/content/concise-guide-monoamine-oxidase-inhibitors-how-avoid-drug-interactions
- Burke, L. (2009). What to know about taking cold medicine on antidepressants. Verywell Mind. https://www.verywellmind.com/cold-medicine-interactions-with-bipolar-medication-380344
- Zhang, P., et al. (2020). Adverse cardiovascular effects of phenylephrine eye drops combined with atropine: A clinical study. Frontiers in Pharmacology, 11, 596539. https://www.frontiersin.org/articles/10.3389/fphar.2020.596539/full
- Niven, D. J., et al. (2021). Adverse events with vasopressor administration through peripheral IVs: A systematic review. Critical Care, 25, 146. https://ccforum.biomedcentral.com/articles/10.1186/s13054-021-03553-1
- Drugs.com. (2024). Phenylephrine use during pregnancy. https://www.drugs.com/pregnancy/phenylephrine.html
- Drugs.com. (2025). Phenylephrine dosage guide. https://www.drugs.com/dosage/phenylephrine.html
- American Society of Health-System Pharmacists. (2023). Phenylephrine hydrochloride injection (AHFS 12:12). https://publications.ashp.org/previewpdf/display/book/9781585286850/ch314.xml
- Gupta, R., et al. (2023). Evaluation of phenylephrine stability in 0.9 % sodium chloride polyvinyl bags. Journal of Pharmaceutical Care Research, 5(2), 45-52. https://juniperpublishers.com/jpcr/pdf/JPCR.MS.ID.555768.pdf
- Schmidt, H., & Ebmeyer, U. (2010). Photostability of phenylephrine hydrochloride. Journal of Pharmaceutical and Biomedical Analysis, 52(7), 789-795. https://www.sciencedirect.com/science/article/pii/S0731708510000270
- Harper, L. M., et al. (2020). Phenylephrine push before continuous infusion in septic shock. Chest, 158(3), 1234-1241. https://journal.chestnet.org/article/S0012-3692%2820%2935353-8/fulltext
- Nguyen, T., et al. (2024). Phenylephrine infusion and postpartum blood loss. AJOG MFM, 6(3), 100319. https://www.ajogmfm.org/article/S2589-9333%2824%2900319-7/fulltext
- Fernandes, R., & Silva, M. (2024). Vasoconstriction with phenylephrine increases cardiac output. Journal of Clinical Monitoring and Computing, 38(4), 701-712. https://link.springer.com/article/10.1007/s10877-024-01186-7
- Meng, L., et al. (2025). Phenylephrine and the risk of atrial fibrillation in critically ill adults. Frontiers in Pharmacology, 16, 1478961. https://www.frontiersin.org/articles/10.3389/fphar.2025.1478961/full
- Smith, A. J., et al. (2016). Safety of peripheral phenylephrine infusion. Journal of Critical Care, 34, 89-94. https://www.traumayellow.com/uploads/2/0/9/5/20955098/safety_of_peripheral_administration_of_phenylephrine.pdf
- InpharmD. (2021). Vasopressor use through peripheral IV lines: Updated literature. https://inpharmd.com/inquiries/46ecffc15f260bcd8315791a5180c48fbe515d9c5fd9a6ed25a1e5d2e018075d
- Pu, Y., et al. (2021). Vasorelaxant effects on phenylephrine-induced contraction. Frontiers in Pharmacology, 12, 695530. https://www.frontiersin.org/articles/10.3389/fphar.2021.695530/pdf
- Badiwala, R., et al. (2014). Intracerebral hemorrhage associated with oral phenylephrine: A case report. Journal of Stroke & Cerebrovascular Diseases, 23(8), 2015-2020. https://www.strokejournal.org/article/S1052-3057%2814%2900204-3/fulltext
Why choose phenylephrine over norepinephrine for short hypotensive episodes during anesthesia?
Phenylephrine offers rapid, predictable increases in arterial pressure without tachyarrhythmia, making it convenient for transient vasodilatory drops.[14]
Can the injection be prepared in normal saline bags ahead of time?
Stability studies show ≤ 1 % degradation for up to 14 days when diluted in 0.9 % sodium chloride and protected from light, but institution-specific beyond-use dating applies.[15]
How quickly should blood pressure rise after an intravenous bolus?
Pressor effect typically begins within 30 seconds and peaks by 2 minutes; reassess before repeating doses to avoid overshoot.[16]
Is continuous infusion safe postpartum?
Controlled trials indicate that titrated infusions maintain maternal pressure and may reduce blood loss after cesarean delivery when used judiciously.[17]
Does phenylephrine always decrease cardiac output?
In preload-dependent states it may paradoxically increase stroke volume by restoring venous return, underlining the importance of volume status assessment.[18]
Could it trigger atrial fibrillation in the ICU?
Large cohort analyses report a modest but significant rise in new-onset atrial fibrillation, necessitating rhythm monitoring in susceptible patients.[19]
Is peripheral administration acceptable?
When diluted to ≤ 40 µg/mL and infused through a well-situated upper-extremity cannula, severe local complications are uncommon, but frequent site checks are essential.[20]
What do recent meta-analyses say about vasopressor extravasation risk?
Aggregated data estimate serious tissue injury in < 1 % of peripheral phenylephrine infusions, supporting its cautious use outside central access when benefits outweigh risks.[21]
How does phenylephrine compare to other vasoconstrictors in pharmacologic research models?
It remains a standard reference agent for inducing smooth-muscle contraction and assessing vasorelaxant compounds in pre-clinical studies.[22]
Are strokes linked to oral or nasal phenylephrine relevant to injectable use?
Case reviews document hemorrhagic events after high systemic exposure; adhering to recommended intravenous doses and vigilant monitoring mitigates such risks.[23]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
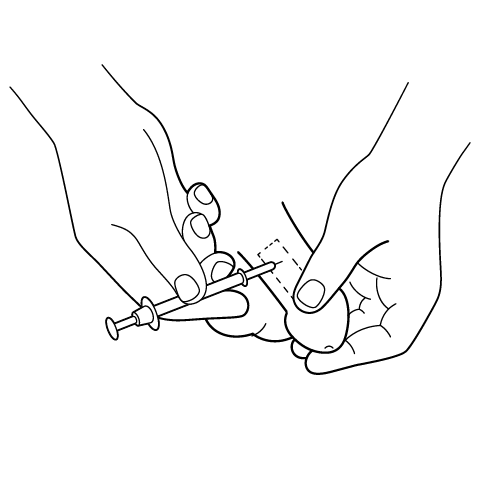
Penile Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.


 Diphenhydramine Injection
Diphenhydramine Injection Dexamethasone Injection
Dexamethasone Injection Quad-Mix Injection
Quad-Mix Injection Tri-Mix Injection
Tri-Mix Injection Bi-Mix Injection
Bi-Mix Injection Testosterone Cypionate Injection
Testosterone Cypionate Injection Anastrozole Capsules
Anastrozole Capsules