Product Overview
† commercial product
Ketorolac Tromethamine Injection is a sterile, aqueous formulation containing the nonsteroidal anti-inflammatory drug (NSAID) ketorolac tromethamine at a concentration of 15 mg per mL in a preservative-free 1 mL vial intended for single-use parenteral administration. As a member of the heteroaryl acetic acid class, the agent exhibits potent analgesic activity comparable with moderate-dose opioids yet retains the anti-inflammatory and antipyretic properties characteristic of prostaglandin G/H synthase (cyclo-oxygenase) inhibitors. The injectable preparation is distributed through an FDA-registered 503B outsourcing facility that operates under current good manufacturing practice (cGMP) requirements, providing prescribers with a ready-to-administer product when commercial supply interruptions or individualized dosing requirements necessitate compounded alternatives.
Clinicians employ the medication for short-term management of moderately severe acute pain following surgical and nonsurgical procedures, usually in hospital or ambulatory surgery center settings where parenteral dosing allows rapid onset of analgesia and reduces reliance on systemic opioids. Despite its efficacy, therapy is deliberately restricted to a maximum cumulative duration of five days in adults because prolonged systemic exposure is associated with a rising incidence of gastrointestinal bleeding, renal dysfunction, and cardiovascular adverse events. Because platelet aggregation is reversibly inhibited for approximately 24 hours after each dose, practitioners must weigh hemorrhagic risk when planning neuraxial anesthesia or postoperative anticoagulation. Proper patient selection-including avoidance in those with active peptic ulcer disease, advanced renal impairment, uncontrolled bleeding disorders, or hypersensitivity to aspirin-like drugs-optimizes the benefit-to-risk profile.
The vial contains 10 % ethanol and isotonic sodium chloride in water for injection; pH is adjusted to 6.9-7.9 with sodium hydroxide or hydrochloric acid. No bacteriostatic agents are present, so unused solution should be discarded.Clinical use is limited to adults ≥17 years because safety and efficacy data in pediatric and chronic pain populations remain insufficient. Prescribers are reminded that compounding does not confer FDA approval and that each prescription must be patient-specific or covered by a valid office-use agreement compliant with state and federal regulations.[1]
For adults 17 years and older weighing ≥50 kg with normal renal function, the recommended initial intramuscular dose is 60 mg once, followed by 30 mg every 6 hours as needed, not to exceed 120 mg in 24 hours. Intravenous bolus dosing is 30 mg every 6 hours with the same daily maximum. Patients ≥65 years, those weighing <50 kg, or those with mild renal impairment should receive half these amounts-30 mg IM or 15 mg IV initially, then 15 mg every 6 hours, with a 60 mg total daily ceiling.
The combined duration of parenteral and any subsequent oral or intranasal ketorolac must not exceed five consecutive days to limit serious toxicity. No pediatric dosing is approved. In severe pain settings, ketorolac may precede a reduced opioid dose, but scheduled, around-the-clock administration rather than on-demand use maintains consistent plasma concentrations and avoids end-of-dose failure. Renal function, hemoglobin concentration, and signs of bleeding should be evaluated daily. Dose adjustments are mandatory for creatinine clearance <50 mL/min, and therapy is contraindicated if clearance falls below 30 mL/min.[7]
Ketorolac’s principal pharmacodynamic effect derives from competitive, reversible inhibition of the cyclo-oxygenase isoenzymes COX-1 and COX-2, thereby blocking the conversion of arachidonic acid to prostaglandin H2, the obligate precursor of pro-inflammatory prostaglandins, prostacyclin, and thromboxanes. Although the racemic mixture has similar total plasma clearance, the S-enantiomer contributes the majority of analgesic potency through high-affinity binding to the COX active site. Peak analgesia follows intravenous or intramuscular administration within 30 to 60 minutes, with a terminal elimination half-life averaging 5.3 hours in healthy adults. Protein binding exceeds 99 %, and the compound distributes into synovial fluid where sustained COX blockade correlates with continued pain relief. Hepatic metabolism, primarily via CYP2C9-mediated hydroxylation and glucuronidation, yields inactive metabolites that are renally excreted.
Because COX-1-derived thromboxane A2 supports platelet aggregation and gastrointestinal mucosal integrity, nonselective inhibition explains the agent’s propensity to prolong bleeding time and erode gastric defenses. Conversely, COX-2 inhibition attenuates peripheral sensitization and central nociceptive transmission, accounting for the opioid-sparing analgesic benefits observed in controlled trials. The drug does not interact with μ-opioid receptors, and it exhibits no intrinsic sedative or respiratory depressant activity. Preclinical models demonstrate analgesic synergy between ketorolac and local anesthetics, supporting multimodal postoperative pain protocols. Investigations into enantioselective pharmacokinetics reveal slower clearance of the R-enantiomer, but clinical efficacy is predominantly linked to the S-form, underscoring the mechanistic rationale for limiting duration rather than escalating dose. Importantly, ketorolac does not significantly inhibit the lipooxygenase pathway and therefore does not raise leukotriene concentrations implicated in aspirin-induced asthma, yet bronchospasm remains possible in NSAID-intolerant individuals.[2]
Use of ketorolac tromethamine is contraindicated in patients with known hypersensitivity to NSAIDs, especially those who have experienced asthma, urticaria, or allergic-type reactions after aspirin exposure, because fatal anaphylactoid responses have been reported. Active peptic ulcer disease, recent gastrointestinal hemorrhage or perforation, and a history of recurrent ulceration preclude therapy, as does cerebrovascular bleeding or patients at high risk for hemorrhage including peri-operative coronary artery bypass graft surgery. The injectable formulation is contraindicated in labor and delivery because prostaglandin inhibition may adversely affect fetal circulation and uterine contractility. Advanced renal impairment (serum creatinine >2.5 mg/dL) or volume depletion increases the likelihood of NSAID-induced renal failure; thus, ketorolac must be avoided or used with extreme caution and dose reduction.
Concomitant use with aspirin, other NSAIDs, probenecid, or pentoxifylline heightens gastrointestinal and bleeding toxicity and represents an absolute or relative contraindication. Patients on anticoagulant therapy, those with hemostatic disorders, or incomplete hemostasis should not receive the drug. Pediatric patients, patients requiring chronic analgesia, and those with inflammatory arthritis are excluded because risk outweighs benefit. Finally, intrathecal or epidural administration is strictly prohibited due to alcohol content and risk of neurotoxicity.[3]
Pharmacodynamic and pharmacokinetic interactions necessitate vigilance during ketorolac therapy. Co-administration with other NSAIDs or high-dose salicylates produces additive cyclo-oxygenase inhibition, compounding gastrointestinal erosion and platelet dysfunction. Probenecid markedly reduces renal clearance of ketorolac, doubling systemic exposure and prolonging half-life; concurrent use is therefore contraindicated. Anticoagulants, direct oral anticoagulants, and antiplatelet agents such as clopidogrel synergistically extend bleeding time. Selective serotonin reuptake inhibitors may impair platelet aggregation via serotonin depletion, further elevating hemorrhagic risk.
Methotrexate clearance may fall when NSAIDs compete for renal tubular secretion, increasing marrow and hepatic toxicity. Lithium excretion is similarly diminished, predisposing to neurotoxicity. Renally-eliminated angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers, when combined with ketorolac and diuretics, form a pharmacologic ‘triple whammy’ that can precipitate acute kidney injury in susceptible individuals. Opioid analgesics exhibit pharmacodynamic complementarity, permitting reduced opioid dose; nonetheless, ketorolac lacks cytochrome induction or inhibition potential, so metabolic interactions are minimal. Practitioners should stagger intramuscular injections at different sites when corticosteroids are prescribed to mitigate additive myopathy.[4]
Adverse events associated with ketorolac align with the NSAID pharmacologic class yet occur with greater frequency when therapy exceeds the recommended five-day limit. The most serious reactions are gastrointestinal bleeding, ulceration, and perforation, which can present without prodromal dyspepsia. Clinically significant postoperative hemorrhage, hematoma formation, and epistaxis have been observed, reflecting reversible platelet inhibition. Renal events include elevations in serum creatinine, oliguria, interstitial nephritis, and, rarely, nephrotic syndrome.
Cardiovascular events, such as hypertension, edema, and in rare cases myocardial infarction or stroke, appear related to prostaglandin imbalance and fluid retention. Central nervous system complaints-headache, dizziness, drowsiness-are usually transient. Dermatologic eruptions range from mild rash to severe exfoliative dermatitis and Stevens-Johnson syndrome. Elevated hepatic transaminases occur in up to 1 % of recipients but seldom progress to fulminant failure. Injection-site pain or burning is reported in roughly 20 % of intramuscular doses. Most adverse effects dissipate after cessation, yet life-threatening bleeding mandates immediate intervention.[5]
Ketorolac crosses the placenta and, like other NSAIDs, may induce premature closure of the fetal ductus arteriosus if administered after 30 weeks’ gestation; it can also impair fetal renal perfusion as early as the second trimester, producing oligohydramnios and neonatal renal insufficiency. Accordingly, the agent is classified as category C prior to 30 weeks and category D thereafter.[6]
Epidemiologic studies do not show a clear association with teratogenicity in early pregnancy, yet data are insufficient to exclude risk, and alternative analgesics with superior maternal-fetal safety profiles are preferred.[6]
During labor, inhibition of prostaglandin-mediated uterine contractions may prolong gestation and increase postpartum hemorrhage. Limited quantities have been detected in breast milk; although adverse neonatal events are unlikely after short courses, nursing mothers should monitor for feeding difficulties or bleeding manifestations.[6]
The drug should be avoided in fertility treatments because COX inhibition may interfere with implantation. Obstetric consultations are recommended when inadvertent exposure occurs.[6]
Store Ketorolac Tromethamine Injection vials at controlled room temperature 20 °C to 25 °C (68 °F to 77 °F). Protect from light by retaining vials in the carton until time of use, and discard solution that appears cloudy, displays particulate matter, or develops pronounced yellow discoloration. Freezing may cause precipitation and should be avoided. The formulation is physically and chemically stable for 24 hours after dilution in normal saline or 5 % dextrose for intravenous infusion, but aseptic technique and prompt administration remain prudent. Because the product is preservative-free, any residual volume must be disposed after single patient use.[8]
- U.S. Food and Drug Administration. (2014). Ketorolac Tromethamine Injection, USP [Package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/074802s038lbl.pdf
- DrugBank Online. (2025). Ketorolac: Uses, interactions, mechanism of action. https://go.drugbank.com/drugs/DB00465
- Pfizer. (2025). Ketorolac tromethamine injection: Warnings and precautions. https://www.pfizermedical.com/patient/ketorolac-inj/warnings
- Drugs..com. (2025). Ketorolac and probenecid interactions. https://www.drugs.com/drug-interactions/ketorolac-with-probenecid-1415-0-1941-0.html
- Drugs..com. (2025). Ketorolac side effects: Common, severe, long term. https://www.drugs.com/sfx/ketorolac-side-effects.html
- Drugs..com. (2025). Ketorolac use during pregnancy. https://www.drugs.com/pregnancy/ketorolac.html
- Drugs..com. (2025). Ketorolac dosage guide + max dose, adjustments. https://www.drugs.com/dosage/ketorolac.html
- Pfizer. (2025). Ketorolac tromethamine injection: Storage and handling. https://www.pfizermedical.com/ketorolac-inj/storage-handling
- U.S. Food and Drug Administration. (2021). Current good manufacturing practice-Guidance for human drug compounding outsourcing facilities under section 503B of the FD&C Act. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/current-good-manufacturing-practice-guidance-human-drug-compounding-outsourcing-facilities-under
- University of Illinois Chicago College of Pharmacy. (2022). What evidence is there for the 5-day maximum with the use of ketorolac? https://dig.pharmacy.uic.edu/faqs/2022-2/september-2022-faqs/what-evidence-is-there-for-the-5-day-maximum-with-the-use-of-ketorolac
- GoodRx. (2025). 8 ketorolac interactions you should know about. https://www.goodrx.com/ketorolac/interactions
- DailyMed. (2025). Ketorolac tromethamine injection, solution. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=47ecf0c5-b808-45c2-aa00-ceaae61da3f6
- Kremer, J. M., & colleagues. (2012). Ketorolac use in acute coronary syndromes. The American Journal of Medicine, 125(11), 1133-1139. https://doi.org/10.1016/j.amjmed.2012.09.014
- Brocks, D. R., & Jamali, F. (1992). Clinical pharmacokinetics of ketorolac tromethamine. Clinical Pharmacokinetics, 23(6), 415-427. https://doi.org/10.2165/00003088-199223060-00003
- U.S. Food and Drug Administration. (2013). Ketorolac tromethamine tablets [Package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019645s019lbl.pdf
- U.S. Food and Drug Administration. (2010). Gastrointestinal, bleeding, cardiovascular, and renal risk boxed warning (ketorolac tromethamine). https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022382s000lbl.pdf
- Baxter Healthcare Corporation. (2021). Ketorolac tromethamine injection prescribing information. https://baxterpi.com/pi-pdf/Ketorolac%20PI-October%202021%2007-19-00-4125.pdf
- Patsnap Synapse. (2024). What is the mechanism of ketorolac tromethamine? https://synapse.patsnap.com/article/what-is-the-mechanism-of-ketorolac-tromethamine
- Trissel, L. A. (2017). Handbook on Injectable Drugs (20th ed.). American Society of Health-System Pharmacists. https://publications.ashp.org/previewpdf/display/book/9781585287079/ch234.xml
What evidence supports the strict five-day maximum duration of therapy
Pharmacovigilance reviews demonstrate a steep rise in GI and renal serious adverse events beyond day five, leading regulators to cap total exposure accordingly.[10]
Which common medications pose the greatest interaction danger?
Probenecid, anticoagulants, other NSAIDs, SSRIs, and lithium significantly increase toxicity risk when combined with ketorolac.[11]
Can the injection be used for chronic conditions such as osteoarthritis pain?
No-its risk profile confines use to short-term, acute pain; chronic administration lacks safety data and is contraindicated.[12]
Does ketorolac increase cardiovascular risk like other NSAIDs?
Observational and experimental data suggest a dose-related rise in thrombotic events, warranting caution in ischemic heart disease.[13]
How quickly does the medication begin to work after IV administration?
Onset of meaningful analgesia usually occurs within 30 minutes, correlating with peak S-enantiomer plasma concentrations.[14]
Should renal function be checked before each dose?
Baseline and periodic serum creatinine are recommended, especially in elders or volume-depleted patients, with dose reduction as needed.[15]
What are the most important counseling points for outpatients discharged with an oral continuation course?
Stress the five-day absolute limit, avoidance of alcohol and NSAID co-therapy, and immediate reporting of black stools or hematuria.[16]
Is there an antidote for inadvertent overdose?
Treatment is supportive; ketorolac is not appreciably removed by hemodialysis, so monitoring and symptomatic care are essential.[17]
Does food intake influence efficacy
Oral bioavailability approaches 100 %, but a light meal may delay Tmax by 30 minutes without altering total analgesic effect.[18]
Disclaimer: This compounded medication is prepared under section 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
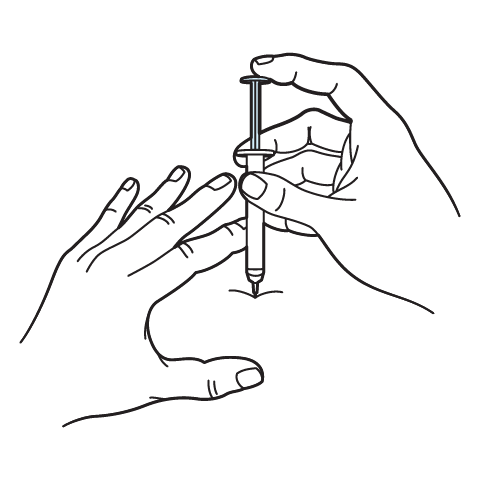
Intramuscular Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Ondansetron Injection
Ondansetron Injection Diphenhydramine Injection
Diphenhydramine Injection Prednisone Tablets
Prednisone Tablets Hydrocortisone Tablets
Hydrocortisone Tablets Dexamethasone Injection
Dexamethasone Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Magnesium Chloride Injection
Magnesium Chloride Injection BCAA Injection
BCAA Injection