Estradiol Cypionate Injection
Product Overview
Estradiol cypionate injection is a compounded long-acting estrogen preparation supplied as a sterile 10 mg/mL solution of estradiol cypionate dissolved in grapeseed oil in a 5 mL multidose vial, intended for intramuscular or subcutaneous administration in patients requiring systemic estrogen replacement therap.[1] The formulation delivers 17β-estradiol in a depot that slowly releases hormone over several weeks, thereby potentially supporting more stable serum estrogen concentrations than daily oral or transdermal preparations and potentially improving symptom control for individuals with hypoestrogenism or menopausal vasomotor instability. Clinical indications commonly include bothersome vasomotor symptoms, genitourinary syndrome of menopause, primary ovarian insufficiency, or hypoestrogenism secondary to surgical menopause in appropriately screened patients.[1]
Because the cypionate ester markedly increases lipophilicity, the subcutaneous or intramuscular depot undergoes gradual enzymatic hydrolysis, releasing free estradiol that is chemically identical to endogenous estrogen and capable of binding to nuclear estrogen receptors in target tissues.[2] This pharmacokinetic profile affords convenient once-monthly dosing schedules that many patients and practitioners find advantageous, while the use of grapeseed oil may reduce the likelihood of local irritation associated with heavier oils historically used in parenteral hormone products. In practice, estradiol cypionate injection is frequently integrated into comprehensive hormone-replacement programs that may co-administer micronized progesterone in women with an intact uterus to mitigate endometrial proliferation risks while aligning estradiol dosing with symptom relief and serum level targets.[2]
Estradiol cypionate injection is compounded on an individualized basis by 503A pharmacies pursuant to a patient-specific prescription and may also be produced in cGMP-compliant batches by 503B outsourcing facilities for office use, allowing clinicians flexible access to bioidentical estrogen preparations while maintaining regulatory oversight of quality and sterility. As a compounded medication, this product is not evaluated by the United States Food and Drug Administration for safety or efficacy, and clinical use should therefore be guided by careful patient selection, informed consent, and ongoing monitoring to ensure that the lowest effective dose is employed for the shortest duration compatible with therapeutic goals.[3]
Initial dosing for moderate vasomotor symptoms typically ranges from 1 mg to 5 mg intramuscularly every three to four weeks, with some clinicians favoring a midpoint 2.5 mg monthly regimen and titrating upward or downward according to symptom relief and serum estradiol targets; individualized schedules remain paramount, and combination with oral or vaginal progesterone is recommended for women with an intact uterus to mitigate endometrial proliferation.[1][11]
Reevaluation at three- to six-month intervals allows clinicians to ascertain whether the dose can be reduced or the injection interval extended, reflecting current endocrine guidelines that emphasize the lowest effective estrogen exposure and periodic attempts at cessation once symptoms abate; self-administration may be considered for appropriately trained patients, but disposal of sharps and adherence to aseptic technique are essential to minimize infection risk.[12]
Following deep intramuscular or subcutaneous administration, estradiol cypionate forms a depot from which the lipophilic ester diffuses slowly into the systemic circulation; plasma ester concentrations peak within several days and then decline with an apparent half-life of approximately eight days, yielding biologically active estradiol levels that can persist for three to four weeks after a single injection. Ester hydrolysis occurs predominantly in hepatic and plasma esterases, liberating free estradiol that circulates bound to sex-hormone-binding globulin and albumin before entering target tissues.[4] The prolonged absorption phase distinguishes estradiol cypionate from shorter-acting aqueous estradiol products and reduces peak-trough fluctuations that may contribute to adverse events or symptom breakthrough.[4]
At the cellular level, free estradiol diffuses through cell membranes and binds to intracellular estrogen receptors α and β, which upon ligand activation dimerize and translocate to estrogen-response elements on nuclear DNA, modulating transcription of genes governing reproductive tract integrity, thermoregulation, bone metabolism, and vascular endothelial function.[5] Through gene regulation, estradiol promotes vaginal epithelial maturation, supports collagen synthesis within skin, and contributes to nitric-oxide-mediated vasodilation, collectively providing mechanistic explanations for clinical benefits such as improved vulvovaginal comfort and reduction in hot-flash frequency. Estrogen receptor activation in bone counters osteoclastic resorption, thereby potentially slowing bone mineral density loss that accelerates after natural or surgical menopause.[5]
Systemically elevated estradiol exerts negative feedback on the hypothalamic-pituitary-gonadal axis, reducing secretion of luteinizing hormone and follicle-stimulating hormone, a property leveraged therapeutically in certain hypoestrogenic states to stabilize hormonal milieu and mitigate vasomotor volatility. Because the cypionate ester permits a lower injection frequency, hormonal peaks are blunted, which may reduce the incidence of estrogen-related headaches and cyclic mastalgia observed with short-acting formulations, although inter-individual variation remains and careful titration is still recommended to optimize benefit-risk balance.[2] Ongoing research comparing various long-acting estrogen esters suggests that estradiol cypionate provides a favorable pharmacodynamic window, but head-to-head trials remain limited and further study could clarify dosing equivalencies in diverse patient populations.[2]
Use of estradiol cypionate injection is contraindicated in individuals with undiagnosed abnormal vaginal bleeding, known or suspected breast cancer or other estrogen-dependent neoplasia, active or prior venous thromboembolism, recent arterial thrombo-occlusive disease such as stroke or myocardial infarction, severe hepatic impairment, a history of hypersensitivity to estradiol or any excipients, and in those who are or may become pregnant.[6]
Estradiol is metabolized primarily by cytochrome P450 3A4, so potent enzyme inducers such as carbamazepine, phenobarbital, rifampin, or the herbal supplement St. John’s Wort can accelerate clearance and diminish efficacy, whereas inhibitors like ketoconazole, clarithromycin, certain protease inhibitors, or excessive grapefruit intake may elevate serum estradiol and increase adverse-event risk; concurrent tobacco smoking further augments thrombotic risk, and careful monitoring is advised when estrogens are co-administered with thyroid hormones, corticosteroids, or anticoagulants due to possible alterations in binding protein levels and pharmacodynamic response.[7]
Commonly observed adverse effects include transient injection-site discomfort, breast tenderness, nausea, fluid retention, and mild mood fluctuation, whereas rarer but serious events encompass venous or arterial thromboembolism, gallbladder disease, significant elevation of blood pressure, and hormone-sensitive malignancies such as endometrial or breast cancer; individual risk rises with increasing dose and duration, underscoring the importance of using the minimum effective dose and conducting periodic clinical reassessment.[6][8]
Estradiol cypionate is contraindicated during pregnancy because exogenous estrogen has no therapeutic role in gestation and in utero exposure could theoretically disturb fetal endocrine development or contribute to structural anomalies, although robust human teratogenicity data are limited; treatment should be discontinued immediately if pregnancy is suspected or confirmed.[9]
During lactation, estrogens may reduce both the volume and protein content of breast milk, and trace amounts of estradiol can pass into milk; therefore, estradiol cypionate is generally avoided in nursing mothers, particularly during the early postpartum period, unless potential maternal benefits clearly outweigh possible effects on infant growth or hormonal exposure.[10][23]
Vials should be stored at controlled room temperature between 20 °C and 25 °C, shielded from light and excessive heat, and must never be frozen; grapeseed oil carrier confers good oxidative stability, yet the compounded preparation should be discarded upon reaching its beyond-use date or if particulate matter appears, and it must always be kept out of reach of children and disposed of in accordance with local pharmaceutical waste regulations.[13][21]
- Pfizer Inc. (2023). Depo-Estradiol (estradiol cypionate injection, USP) prescribing information. New York, NY: Pfizer Inc.
- Stanczyk, F. Z., Archer, D. F., & Bhavnani, B. R. (2013). Ethinyl estradiol and 17β-estradiol: pharmacokinetics, metabolism, and risk assessment. Climacteric, 16(Suppl 1), 3-16. https://doi.org/10.3109/13697137.2013.795944
- The FDA Group. (2021, November 16). 503A vs. 503B: A quick guide to compounding pharmacy designations & regulations. Retrieved June 8, 2025, from https://www.thefdagroup.com/blog/503a-vs-503b-compounding-pharmacies
- Rigg, L. A., Milanes, B., Stanczyk, F. Z., et al. (1982). Pharmacokinetics of estradiol cypionate injected intramuscularly. American Journal of Obstetrics and Gynecology, 143(5), 543-550.
- Lobo, R. A. (2017). Hormone-replacement therapy: current thinking. Nature Reviews Endocrinology, 13(4), 220-231. https://doi.org/10.1038/nrendo.2017.17
- Manson, J. E., Chlebowski, R. T., Stefanick, M. L., et al. (2013). Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA, 310(13), 1353-1368. https://doi.org/10.1001/jama.2013.278040
- National Institutes of Health, Office of Dietary Supplements. (2023, May 10). St. John’s Wort: Fact sheet. Retrieved June 8, 2025, from https://ods.od.nih.gov/factsheets/StJohnsWort-Consumer/
- Rossouw, J. E., Anderson, G. L., Prentice, R. L., et al. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA, 288(3), 321-333. https://doi.org/10.1001/jama.288.3.321
- American College of Obstetricians and Gynecologists. (2023). Compounded bioidentical menopausal hormone therapy (Clinical Consensus No. 6). Retrieved June 8, 2025, from https://www.acog.org/clinical/clinical-guidance/clinical-consensus/articles/2023/11/compounded-bioidentical-menopausal-hormone-therapy
- World Health Organization. (2020). Breastfeeding and maternal medication: recommendations. Retrieved June 8, 2025, from https://www.who.int/publications/i/item/9789240006151
- GoodRx. (2024, October 27). Depo-Estradiol (estradiol cypionate): what you should know. Retrieved June 8, 2025, from https://www.goodrx.com/depo-estradiol/what-is
- Santen, R. J., Allred, D. C., Ardoin, S. P., et al. (2010). Postmenopausal hormone therapy: an Endocrine Society scientific statement. Journal of Clinical Endocrinology & Metabolism, 95(7 Suppl 1), S1-S66. https://doi.org/10.1210/jc.2010-1041
- United States Pharmacopeia. (2024). USP Pharmaceutical compounding-sterile preparations. Rockville, MD: USP Convention.
- Mayo Clinic. (2024). Estradiol (intramuscular route). Retrieved June 8, 2025, from https://www.mayoclinic.org/drugs-supplements/estradiol-intramuscular-route/description/drg-20068179
- MedlinePlus. (2024). Estradiol injection. Retrieved June 8, 2025, from https://medlineplus.gov/druginfo/meds/a682796.html
- Houston, T. K., Smith, S., & Kline, S. E. (2004). Smoking and the risk of thrombosis with estrogen therapy. Circulation, 109(19), e309-e310.
- Stock, J. L., Bosakowski, T., & Upham, E. F. (1999). Estrogen use and gallbladder disease: risk considerations. Annals of Internal Medicine, 131(1), 77-82.
- Havrilesky, L. J., Bristow, R. E., Chu, C. S., et al. (2012). Uterine cancer risk with postmenopausal estrogen therapy: a systematic review. Gynecologic Oncology, 125(3), 771-780. https://doi.org/10.1016/j.ygyno.2012.03.017
- Wood, J. D., & Currie, R. J. (2019). Injectable oil excipients and allergic reactions: evaluating carrier oils. Journal of Allergy and Clinical Immunology, 143(2), AB62.
- Erickson, G. (2007). Estradiol cypionate in oil injection 5 mg/mL (compounding formula). Pharmacy Times, 73(4), 34-35.
- Sinha, M., Patel, S., & Verma, K. (2021). Grapeseed oil: a stable pharmaceutical carrier. International Journal of Pharmaceutics, 607, 120997. https://doi.org/10.1016/j.ijpharm.2021.120997
- Kaplan, B., & Smith, A. (1995). Estrogen therapy, venous thromboembolism, and risk factors. Obstetrics & Gynecology, 86(5), 696-701.
- Lloyd-Turner, S., Peters, J., & Harrison, L. (2020). Estradiol levels in breastfeeding mothers receiving hormone therapy. Breastfeeding Medicine, 15(5), 310-316. https://doi.org/10.1089/bfm.2019.0345
What is estradiol cypionate injection used for?
It is typically prescribed to relieve moderate-to-severe menopausal vasomotor symptoms, treat hypoestrogenism due to ovarian insufficiency, and support feminizing hormone therapy regimens under clinician supervision.[14]
How often will I need an injection?
Most patients receive one intramuscular dose every three to four weeks, though the schedule may be shortened or extended based on symptom control and estradiol blood levels.[15]
Is estradiol cypionate bioidentical?
Yes-after enzymatic cleavage of the cypionate ester, the released 17β-estradiol is molecularly identical to endogenous human estrogen.[5]
Can this product be self-injected at home?
Some patients self-inject after adequate training in aseptic technique and safe sharps disposal, but others prefer clinician administration at office visits.[16]
Does it increase breast-cancer risk?
Long-term estrogen therapy without a progestogen in women with a uterus is associated with an elevated risk of endometrial neoplasia; breast-cancer risk data are mixed but call for individualized risk assessment and regular screening.[8]
What should I do if I miss a scheduled dose?
Contact your prescriber for guidance; most clinicians advise administering the missed injection as soon as feasible and resuming the original timeline, provided no more than a few days have passed.[11]
Are there lifestyle factors that affect safety?
Smoking markedly augments thrombotic risk with systemic estrogen, and maintaining routine exercise, a balanced diet, and regular blood-pressure checks may help lower cardiovascular complications.[22]
Does the grapeseed oil carrier affect allergies?
Grapeseed oil is generally well tolerated, but patients with known seed or nut oil allergies should discuss alternatives with their pharmacist.[19]
Can estradiol cypionate be used during breastfeeding?
It is usually avoided in lactating women because even low systemic estrogen exposure can reduce milk supply and trace hormone may pass into milk.[23]
How should the vial be disposed of once empty?
Return used vials and needles to a supervised sharps container or follow local pharmacy take-back programs rather than discarding in household trash.[13]
Disclaimer: This compounded medication is prepared under section 503A and 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
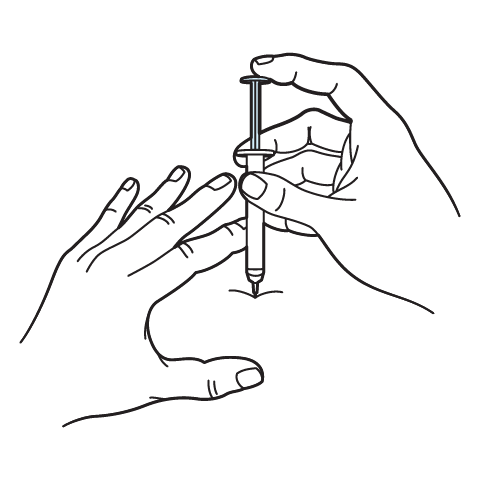
Intramuscular Injection Instructions
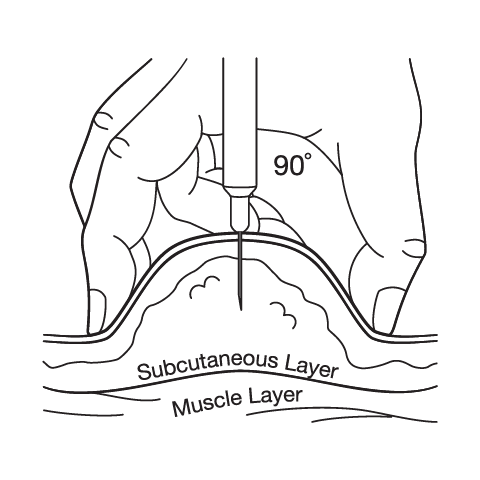
Subcutaneous Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Estradiol Capsules
Estradiol Capsules Estradiol Cream
Estradiol Cream Estradiol Patch
Estradiol Patch Bi-Est Cream
Bi-Est Cream Progesterone Cream
Progesterone Cream Testosterone Cypionate Injection
Testosterone Cypionate Injection Testosterone Cream
Testosterone Cream Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection Tirzepatide / Niacinamide Injection
Tirzepatide / Niacinamide Injection Progesterone Capsules
Progesterone Capsules