Product Overview
† commercial product
Diphenhydramine Injection is a sterile, preservative-free parenteral preparation containing 50 mg of diphenhydramine hydrochloride per mL, adjusted to pH 4-6.5 with hydrochloric acid or sodium hydroxide and supplied in single-dose 1 mL vials intended for deep intramuscular or slow intravenous administration.[1]
As a first-generation ethanolamine antihistamine, the formulation is used when oral therapy is impossible or impractical to manage acute IgE-mediated reactions, adjunctive treatment of anaphylaxis (after epinephrine), prophylaxis of blood-product hypersensitivity, motion sickness that precludes enteral dosing, and selected extrapyramidal syndromes.[2]
The drug distributes widely, crosses the blood-brain barrier, and produces rapid onset of action-often within minutes after IV push-because high lipophilicity accelerates central nervous system penetration; the resultant anticholinergic and sedative properties underlie many clinical uses and cautions.[3]
Compounded under section 503B, this outsourced product is dispensed by prescription only; it is not FDA-approved but is prepared to current good manufacturing practice standards, undergoes sterility/endotoxin testing, and must be prescribed and monitored by qualified clinicians.[4]
For adults, the usual intramuscular or slow intravenous dose ranges from 10 mg to 50 mg per administration, not to exceed 400 mg in 24 hours; infusion rates should remain below 25 mg min⁻¹ to mitigate transient hypotension.[21]
Current labeling advises 5 mg kg⁻¹ day⁻¹ (or 150 mg m⁻² day⁻¹) divided every 6-8 hours for pediatric patients beyond the neonatal period, with a single-dose maximum of 50 mg; premixing into a minibag and infusing over 10-15 minutes is acceptable for larger cumulative doses and may reduce burning at injection sites.[22]
Diphenhydramine acts as a reversible, competitive antagonist-and more precisely, an inverse agonist-at the histamine H₁ receptor, thereby stabilizing the inactive receptor conformation and preventing histamine-mediated vasodilation, increased capillary permeability, and sensory nerve activation.[5]
Because the molecule readily penetrates the central nervous system, blockade of H₁ receptors in cortical and subcortical arousal pathways diminishes wake-promoting histaminergic signaling, producing dose-dependent somnolence and impairment of attention, memory, and psychomotor speed.[6]
At anticholinergic doses it also antagonizes muscarinic M₁ receptors, potentiating drying of secretions, mydriasis, tachycardia, and decreased gastrointestinal motility; cytochrome P450 isoenzymes (primarily CYP2D6 and 1A2) mediate hepatic N-demethylation, explaining pharmacokinetic variability and drug-drug interaction potential.[7]
Experimental psychometric studies using double-blind crossover designs confirm measurable decrements in sustained attention and reaction time within 0.5-3.5 h after intravenous administration of 50 mg, correlating with cortical H₁ receptor occupancy.[8]
Contemporary reviews emphasize that first-generation agents such as diphenhydramine are inverse agonists rather than simple antagonists and note emerging data on gene-dose effects, transporter interactions, and tolerance development, all of which should temper prolonged off-label use for insomnia.[9]
Parenteral diphenhydramine is contraindicated in premature or full-term neonates because of heightened anticholinergic central toxicity and paradoxical excitation.[10]
The injectable solution must not be used as a local anesthetic or via subcutaneous or intradermal routes owing to reports of local necrosis and limb-threatening extravasation injuries, particularly in fragile pediatric or elderly veins.[11]
Clinicians should avoid administration in patients with known hypersensitivity to ethanolamine antihistamines, narrow-angle glaucoma, symptomatic prostatic hypertrophy, stenosing peptic ulcer, bladder-neck obstruction, or severe chronic obstructive pulmonary disease, as peripheral antimuscarinic effects can precipitate urinary retention, acute angle closure, or bronchial thickening.[12]
Concomitant use with central nervous system depressants-including benzodiazepines, opioids, barbiturates, alcohol, or cannabis-may produce additive sedation, impaired airway reflexes, and respiratory depression; therefore dosing intervals should be staggered or alternative non-sedating antihistamines selected.[13]
Monoamine oxidase inhibitors prolong and intensify diphenhydramine’s anticholinergic actions by decreasing metabolic clearance and augmenting neurotransmitter synaptic levels, so co-administration (or administration within 14 days of MAOI discontinuation) is discouraged.[14]
In vitro and clinical pharmacology data further demonstrate potentiation of xerostomia, hyperthermia, and tachyarrhythmia when combined with other anticholinergic agents such as tricyclic antidepressants or phenothiazines; caution with polypharmacy in geriatric populations is warranted.[15]
Common adverse effects encompass dose-related somnolence, dizziness, disturbed coordination, and mucosal dryness; cardiovascular reactions such as hypotension and palpitations occur less frequently but mandate monitoring when large intravenous boluses (≥ 50 mg) are delivered over < 2 minutes.[16] Large pharmacoepidemiologic reviews and recent randomized trials link first-generation H₁ antihistamines to impaired driving performance, increased fall risk in adults > 65 years, and paradoxical agitation, hallucinations, or seizures in children, underscoring the need for individualized risk-benefit assessment.[17]
Animal reproduction studies at up to five times the human dose revealed no teratogenicity, and epidemiologic surveillance classifies diphenhydramine as pregnancy category B; nevertheless, absence of controlled human trials dictates cautious, short-term use only when expected benefits outweigh fetal risks.[18]
Obstetric pharmacologists emphasize potential neonatal respiratory depression if high maternal doses are administered during labor and recommend avoidance of elective parenteral administration within two weeks of expected delivery unless medically essential.[19]
During lactation, diphenhydramine appears in breast milk at low concentrations, but sedative transfer may blunt infant arousal or theoretically diminish milk production; non-sedating second-generation antihistamines are generally preferred for chronic allergy management in breastfeeding parents.[20]
Store vials at controlled room temperature 20-25 °C (68-77 °F); protect from light, avoid freezing, retain in carton until use, and discard any unused portion immediately after entry to prevent microbial contamination of this preservative-free solution.[23]
- U.S. Food and Drug Administration. (2013). Diphenhydramine Hydrochloride Injection, USP [Package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/091526lbl.pdf
- Cleveland Clinic. (2025). Diphenhydramine Injection. https://my.clevelandclinic.org/health/drugs/18233-diphenhydramine-injection
- Pharmacology Mentor. (2024). Pharmacology of diphenhydramine. https://pharmacologymentor.com/pharmacology-of-diphenhydramine/
- Drugs..com. (2025). Diphenhydramine Injection: Prescribing information. https://www.drugs.com/pro/diphenhydramine-injection.html
- Simons, P. J., & Simons, K. J. (1994). Mechanisms of antihistamine-induced sedation in the human brain: H1 receptor involvement. Neuroscience, 59(3), 499-513. https://doi.org/10.1016/0306-4522(94)90178-3
- Wikipedia contributors. (2025). H1 antagonist. In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/H1_antagonist
- Indiana University School of Medicine. (n.d.). Cytochrome P450 drug interaction table. https://drug-interactions.medicine.iu.edu/Main-Table.aspx
- Kay, G. G., et al. (2003). Sedation and performance impairment of diphenhydramine and second-generation antihistamines. Journal of Allergy and Clinical Immunology, 111(5), 770-776. https://doi.org/10.1067/mai.2003.1292
- Wallace, D. V., et al. (2023). Evidence-based use of antihistamines for allergic disease. Annals of Allergy, Asthma & Immunology, 131(4), 412-425. https://doi.org/10.1016/j.anai.2023.02.017
- .BD Rx Inc. (2025). Diphenhydramine hydrochloride injection, NDC 63323-664 [Label]. https://ndclist.com/ndc/63323-664/label
- Royal Children’s Hospital Melbourne. (n.d.). Peripheral extravasation injuries: Management guideline. https://www.rch.org.au/clinicalguide/guideline_index/Peripheral_Extravasation_Injuries/
- Verywell Health. (2009). Benadryl: Side effects and cautions. https://www.verywellhealth.com/benadryl-medication-information-83082
- National Health Service. (2025). Taking diphenhydramine with other medicines. https://www.nhs.uk/medicines/diphenhydramine/taking-or-using-diphenhydramine-with-other-medicines-and-herbal-supplements/
- Drugs..com. (2025). Diphenhydramine-Drug interactions. https://www.drugs.com/pro/diphenhydramine.html
- Asian Journal of Pharmaceutical Technology. (2024). Exploring the spectrum of diphenhydramine pharmacological mechanisms and safety considerations. https://www.asianpharmtech.com/articles/exploring-the-spectrum-of-diphenhydramine-pharmacological-mechanisms–clinical-indications-and-safety-considerations.pdf
- Drugs..com. (2024). Diphenhydramine: Side effects. https://www.drugs.com/sfx/diphenhydramine-side-effects.html
- Beaucage-Charron, M., et al. (2023). Side effects of antihistamines. Toxicology Letters, 380, 15-22. https://doi.org/10.1016/S0378-6080(23)00029-6
- Drugs..com. (2025). Diphenhydramine use during pregnancy. https://www.drugs.com/pregnancy/diphenhydramine.html
- National Health Service. (2025). Diphenhydramine: Pregnancy and breastfeeding. https://www.nhs.uk/medicines/diphenhydramine/pregnancy-breastfeeding-and-fertility-while-taking-or-using-diphenhydramine/
- DrLact. (2024). Diphenhydramine and breastfeeding. https://drlact.com/drug/96/diphenhydramine-in-breastfeeding
- Drugs..com. (2025). Benadryl injection dosage guide. https://www.drugs.com/dosage/benadryl-injection.html
- DailyMed. (2025). Diphenhydramine hydrochloride injection (Set ID 921b9f64-1096-46db-866a-67f0f9ff6790). https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=921b9f64-1096-46db-866a-67f0f9ff6790
- Merck. (2023). Diphenhydramine hydrochloride solution safety data sheet. https://gps.mylwebhub.com/…/DIPHENHYDRAMINE_HYDROCHLORIDE_SFI_MTR_NGHS_EN.ashx
- Drugs..com. (2025). How long does Benadryl take to work? https://www.drugs.com/medical-answers/long-benadryl-work-3573470/
- .HealthyChildren.org. (2025). Diphenhydramine dosing table. https://www.healthychildren.org/…/Diphenhydramine-Benadryl-Antihistamine.aspx
- Verywell Health. (2025). Is Benadryl safe for children? https://www.verywellhealth.com/benadryl-for-kids-11722743
- RxList. (2023). Benadryl injection: Uses and side effects. https://www.rxlist.com/benadryl-injection-drug.htm
- American Society of Health-System Pharmacists. (2021). Adult quick IV push reference table. https://www.med.unc.edu/…/Adult-Quick-IV-Push-ED-Reference-Table.pdf
- RxReasoner. (2024). Nytol One-A-Night: Precautions and interactions. https://www.rxreasoner.com/monographs/nytol/precautions
- DrugToday Online. (2024). Diphenhydramine: Uses, dosage, interactions. https://www.drugtodayonline.com/drug-info/diphenhydramine
- Verywell Health. (2023). How much Benadryl can I take? https://www.verywellhealth.com/how-much-benadryl-can-i-take-7499814
- Children’s Hospital of Eastern Ontario. (2024). Diphenhydramine IV compatibility. https://outreach.cheo.on.ca/manual/1552
- Trissel, L. A. (2023). Extended stability for parenteral drugs (7th ed.). American Society of Health-System Pharmacists.
How quickly does the injection work?
Intravenous administration usually produces clinical effect within minutes, with peak antihistaminic activity reached in 5-10 minutes.[24]
What is the weight-based pediatric dose?
For children ≥ 2 years, 0.5-1 mg kg⁻¹ every 6-8 hours up to 5 mg kg⁻¹ day⁻¹ is typical, never exceeding 50 mg per single dose.[25]
Is it safe in young children?
Children under 2 years should not receive diphenhydramine without specialist oversight because of reported paradoxical excitation and respiratory events.[26]
Besides allergies, what can this injection treat?
It may be used for acute dystonic reactions from antipsychotics, motion sickness when oral dosing is not possible, and adjunctive management of anaphylaxis once epinephrine is given.[27]
Can it be pushed through a central line?
Yes but administer slowly (≤ 25 mg min⁻¹) and flush well; ensure compatibility with co-infused solutions to avoid precipitation.[28]
Does it interact with herbal sleep aids?
Products containing valerian or kava may synergistically increase central sedation and should be avoided when parenteral diphenhydramine is planned.[29]
What about combining with other antihistamines?
Stacking first-generation agents raises anticholinergic burden and confers no added efficacy; consider a modern non-sedating H₁ blocker instead.[30]
What is the absolute maximum daily dose?
Do not exceed 400 mg in adults within 24 hours, as higher exposure correlates with delirium, seizures, and cardiotoxicity.[31]
Is the drug compatible with normal saline?
Yes; stability studies show compatibility with 0.9 % sodium chloride, D5W, and lactated Ringer’s, but admixtures should be used within 24 hours and protected from light.[32]
Disclaimer: This compounded medication is prepared under section 503B of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
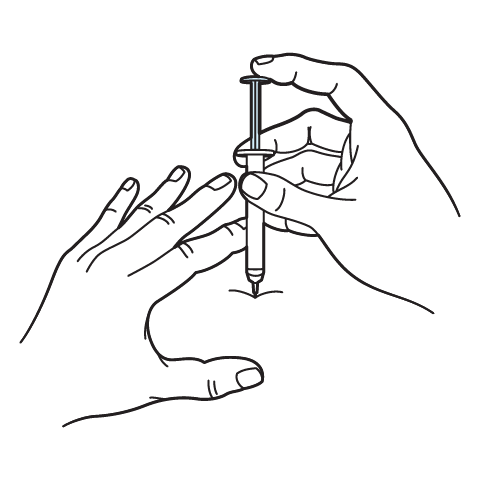
Intramuscular Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Ondansetron Injection
Ondansetron Injection Ketorolac Tromethamine Injection
Ketorolac Tromethamine Injection Prednisone Tablets
Prednisone Tablets Hydrocortisone Tablets
Hydrocortisone Tablets Dexamethasone Injection
Dexamethasone Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Glutathione Injection
Glutathione Injection BCAA Injection
BCAA Injection