Product Overview
† commercial product
Dexamethasone is a potent, long-acting synthetic fluorinated glucocorticoid first described in the late 1950s and adopted for parenteral therapy soon afterward. As the 16-methyl analogue of cortisol, it possesses roughly 25-fold greater anti-inflammatory potency yet virtually no mineralocorticoid activity, allowing clinicians to achieve strong glucocorticoid effects without problematic sodium retention. The injectable preparation is formulated as the highly water-soluble sodium-phosphate ester, enabling rapid systemic distribution after intravenous (IV) or intramuscular (IM) administration.[1]
The 4 mg / mL single-use vial widely stocked by pharmacies contains dexamethasone sodium phosphate in an isotonic, phosphate-buffered vehicle that is preservative-free and compatible with common diluents.[2]
Injectable dexamethasone appears on the World Health Organization Model List of Essential Medicines and is considered a standard of care for cerebral edema, acute asthma exacerbations, spinal cord compression, and adrenal crisis. Its profile was further elevated when the RECOVERY trial demonstrated a mortality benefit in hospitalized adults with COVID-19 receiving oxygen or mechanical ventilation.[3]
Beyond critical care, the agent is integral to protocols across rheumatology, hematology-oncology, neurosurgery, and allergy, where its 36- to-48-hour biologic half-life provides durable symptom control. Patients often report marked reductions in pain, emesis, and airway obstruction within 24 hours, while hemodynamic stability distinguishes it from shorter-acting steroids.[4]
The drug’s low acquisition cost-often under one U.S. dollar per vial-helped sustain global supply during the pandemic, and post-hoc health-economic analyses attribute tens of thousands of prevented deaths to its availability.[5]
Because the active ingredient is off-patent, outsourcing facilities and traditional 503A pharmacies may repackage or customize concentration and fill-volume. These compounded preparations are prescription-only and should not be presumed therapeutically identical to FDA-approved products without clinician verification.[6]
Typical adult anti-inflammatory dosing is 0.04-0.2 mg/kg IV or IM (≈ 3-15 mg for a 70 kg patient), with limited benefit above 20 mg/day outside oncologic or neurologic emergencies.[27]
Septic-shock and ARDS protocols use 0.1 mg/kg daily up to ten days, whereas perioperative antiemetic regimens commonly employ a single 4-10 mg IV bolus at induction, capitalizing on rapid onset (< 10 min IV) and 36-hour biologic half-life.[28]
Pediatric dosing of 0.15-0.6 mg/kg IV or IM (max 16 mg) treats croup, asthma, or adrenal emergencies; courses should be limited to ≤ 48 h or tapered to prevent adrenal suppression.[29]
Dexamethasone binds with high affinity to the cytosolic glucocorticoid receptor (GR). Ligand binding prompts dissociation from heat-shock proteins, nuclear translocation, and interaction with glucocorticoid response elements, thereby modulating hundreds of genes involved in cytokine synthesis, cellular adhesion, and apoptosis. Structural studies show that 9-fluoro and 16-methyl substitutions enhance receptor affinity and prolong residence time.[7]
Transcriptomic experiments reveal down-regulation of NF-κB and AP-1 alongside up-regulation of anti-inflammatory mediators such as annexin-1, culminating in reduced prostaglandin, leukotriene, and nitric-oxide synthesis.[8]
Hematologically the drug causes transient neutrophilic demargination, lymphopenia due to T-cell redistribution, and functional inhibition of antigen-presenting cells, explaining both its therapeutic anti-inflammatory action and its predisposition to infection when therapy is prolonged.[9]
Pharmacokinetic analyses indicate that dexamethasone is a CYP3A4 substrate and auto-inducer, increasing the expression of metabolic enzymes and efflux transporters-factors that complicate dosing when multiple CYP3A modulators are present.[10]
Non-genomic actions, including membrane-bound GR modulation of MAP-kinase signaling and stabilization of lysosomal membranes, account for the rapid relief some patients experience within minutes of IV administration.[11]
Active, untreated systemic fungal infection constitutes an absolute contraindication because the drug’s immunosuppressive properties can accelerate dissemination.[12]
Use is also contraindicated in patients with known hypersensitivity to dexamethasone or formulation excipients; anaphylactoid reactions, though rare, have been documented.[13]
Relative contraindications include uncontrolled diabetes, severe hypertension, and active peptic ulcer disease, in which glucocorticoid-induced metabolic or hemodynamic shifts may provoke acute deterioration.[14]
Because dexamethasone lacks mineralocorticoid activity, it should not replace hydrocortisone in primary adrenal insufficiency, and its administration must not delay physiologic steroid replacement when that diagnosis is suspected.[15]
CYP3A4 inducers-carbamazepine, phenytoin, St. John’s wort-can lower dexamethasone exposure, necessitating dose adjustment for equivalent anti-inflammatory effect.[16]
Rifampin co-administration has produced therapeutic failure of dexamethasone suppression tests and inadequate symptom control in tuberculosis patients.[17]
CYP3A4 induction by rifampin, but not by dexamethasone, reduces bortezomib exposure almost 50 %, compromising oncologic efficacy and illustrating the need for multidisciplinary oversight.[18]
Physiologically based pharmacokinetic models predict that maximal intestinal and hepatic induction occurs after seven days of daily dosing, implying that even brief high-dose courses can alter calcineurin-inhibitor or direct-oral-anticoagulant disposition.[19]
Common transient effects after a single injection include flushing, leukocytosis, insomnia, and dyspepsia, typically resolving within 48 hours.[20]
Psychiatric reactions-mood swings, anxiety, mania, psychosis-may emerge even at modest doses; vigilance and prompt dose modification are imperative when early symptoms arise.[21]
Prolonged or repeated dosing accelerates bone resorption and suppresses osteoblast function, necessitating calcium, vitamin D, and bisphosphonate prophylaxis when cumulative exposure exceeds three months.[22]
Observational data link cumulative dexamethasone exposure with early posterior-subcapsular cataracts, prompting recommendations for periodic ophthalmic evaluation during intermittent high-dose therapy.[23]
Guidelines support a single course of antenatal corticosteroids between 24 + 0 and 33 + 6 weeks’ gestation for women at risk of preterm birth, with clear neonatal benefits for respiratory function and intraventricular-hemorrhage reduction.[24]
A randomized comparison found two 12-mg IM doses of dexamethasone 12 hours apart to be non-inferior to betamethasone for fetal lung maturation, though optimal dosing to minimize neonatal hypoglycemia remains under investigation.[25]
Systematic reviews advise against routine corticosteroid use beyond 34 weeks because of potential neonatal hypoglycemia and neurodevelopmental concerns, highlighting the need for individualized risk-benefit evaluation.[26]
Laboratory studies show > 95 % potency retention for dexamethasone sodium phosphate vials stored 20-25 °C and shielded from intense light for 91 days.[30]
Temperature excursions below 15 °C or above 30 °C hasten hydrolysis of the phosphate ester and may cause visible particulates, so household refrigerators and hot vehicles should be avoided.[31]
Admixtures with ketamine remain stable up to 24 h at 37 °C in polypropylene syringes; beyond this, any haze or discoloration warrants immediate disposal.[32]
- DrugBank Online. (2025). Dexamethasone. https://go.drugbank.com/drugs/DB01234
- Drugs..com. (2025). Dexamethasone injection: Package insert / prescribing information. https://www.drugs.com/pro/dexamethasone-injection.html
- The RECOVERY Collaborative Group. (2021). Dexamethasone in hospitalized patients with COVID-19. New England Journal of Medicine, 384, 693-704. https://doi.org/10.1056/NEJMoa2021436
- Cleveland Clinic. (2025). Dexamethasone injection: Uses & side effects. https://my.clevelandclinic.org/health/drugs/20480-dexamethasone-injection
- Park, A. (2020, June 17). A cheap, common steroid called dexamethasone may help patients with severe COVID-19. Time. https://time.com/5868223/dexamethasone-covid-19-study/
- Drugs..com. (2025). Dexamethasone: Professional monograph. https://www.drugs.com/monograph/dexamethasone.html
- Rhen, T., & Cidlowski, J. A. (2005). Antiinflammatory action of glucocorticoids. Nature Reviews Immunology, 5, 1-15. https://doi.org/10.1038/nri1729
- Fernandes, A. M., et al. (2024). Influence of dexamethasone on GR binding and gene expression. Molecular Immunology, 154, 230-240. https://doi.org/10.1016/j.molimm.2023.10.015
- UpToDate. (2025). Glucocorticoid effects on the immune system. https://www.uptodate.com/contents/glucocorticoid-effects-on-the-immune-system
- Visser, M. E., et al. (2023). Drug-drug interactions involving dexamethasone. Journal of Clinical Medicine, 12, 7120. https://doi.org/10.3390/jcm12227120
- Weber, M., & Weisser, J. (2022). How osteogenic is dexamethasone? Frontiers in Cell and Developmental Biology, 10, 953516. https://doi.org/10.3389/fcell.2022.953516
- DailyMed. (2025). Dexamethasone tablets-drug information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5622f8d2-1893-441b-8011-fa90c8a63b0b
- BC Cancer. (2013). Dexamethasone monograph. https://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Dexamethasone_monograph_1June2013_formatted.pdf
- WikiAnesthesia. (2024). Dexamethasone. https://wikianesthesia.org/wiki/Dexamethasone
- Health Canada. (2024). Dexamethasone sodium phosphate injection USP-product monograph. https://pdf.hres.ca/dpd_pm/00058006.PDF
- UpToDate. (2025). Cytochrome P450 3A inhibitors and inducers. https://www.uptodate.com/contents/image?imageKey=CARD/76992
- Drugs..com. (2025). Dexamethasone and rifampin-drug interaction. https://www.drugs.com/drug-interactions/dexamethasone-with-rifampin-810-0-2012-0.html
- Blood. (2010). Effect of CYP3A4 inducers on bortezomib exposure. Blood, 116, 3983. https://doi.org/10.1182/blood.V116.21.3983.3983
- Ning, J., et al. (2024). PBPK model of rifampicin predicts transporter interactions. CPT: Pharmacometrics & Systems Pharmacology, 13, 289-302. https://doi.org/10.1002/psp4.12807
- Drugs..com. (2025). Dexamethasone-side effects. https://www.drugs.com/sfx/dexamethasone-side-effects.html
- Warrington, T. P., & Bostwick, J. M. (2006). Psychiatric adverse effects of corticosteroids. Mayo Clinic Proceedings, 81, 1361-1367. https://doi.org/10.4065/81.10.1361
- Cedars-Sinai. (2025). Corticosteroid-induced osteoporosis. https://www.cedars-sinai.org/health-library/diseases-and-conditions/c/corticosteroid-induced-osteoporosis.html
- Banerjee, A. J. H., et al. (2024). Dexamethasone and cataract risk in myeloma. Blood, 144(Suppl 1), 7828. https://doi.org/10.1182/blood-2024-144-7828
- American College of Obstetricians and Gynecologists. (2017). Antenatal corticosteroid therapy for fetal maturation (Committee Opinion No. 713). https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/antenatal-corticosteroid-therapy-for-fetal-maturation
- Crowther, C. A., et al. (2019). Maternal intramuscular dexamethasone versus betamethasone. The Lancet Child & Adolescent Health, 3, 803-813. https://doi.org/10.1016/S2352-4642(19)30292-5
- Roberts, D., et al. (2017). Antenatal corticosteroids for accelerating fetal lung maturation. Cochrane Database of Systematic Reviews, 3, CD004454. https://doi.org/10.1002/14651858.CD004454.pub3
- Drugs..com. (2025). Dexamethasone dosage guide. https://www.drugs.com/dosage/dexamethasone.html
- Medicine.com. (2020). Dexamethasone (systemic): Pharmacology & onset. https://www.medicine.com/drug/dexamethasone-systemic/hcp
- U.S. FDA. (2014). Dexamethasone sodium phosphate injection label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/40572s002lbledt.pdf
- Rodgers, P., et al. (1996). Stability of dexamethasone sodium phosphate. PDA Journal of Pharmaceutical Science & Technology, 50, 261-266. https://journal.pda.org/content/50/4/261
- Appliance Update. (2024, Dec 5). Should dexamethasone be refrigerated? https://applianceupdate.com/should-dexamethasone-be-refrigerated/
- Trissel, L. A., et al. (2004). Compatibility of dexamethasone with ketamine. International Journal of Pharmaceutics, 270, 207-215. https://doi.org/10.1016/j.ijpharm.2003.11.017
- Chemocare. (2025). Dexamethasone (injection) patient education. https://chemocare.com/druginfo/dexamethasone-(injection)
- American Family Physician. (1998). A different look at corticosteroids. American Family Physician, 58, 443-450. https://www.aafp.org/pubs/afp/issues/1998/0801/p443.html
- Bruyère, O., et al. (2023). Side effects of corticosteroid injections. AJR, 221, 1058-1065. https://doi.org/10.2214/AJR.23.30458
- Kronzer, K. E., et al. (2022). Dexamethasone and surgical-site infection. New England Journal of Medicine, 387, 1243-1254. https://doi.org/10.1056/NEJMoa2028982
- Singh, R., & Kaur, J. (2014). Pain and itching at dexamethasone injection sites. IOSR-JPBS, 9, 13-14. https://www.iosrjournals.org/iosr-jpbs/papers/Vol9-issue3/Version-5/C09351314.pdf
- Wellwisp. (2025). How quickly does dexamethasone work? https://wellwisp.com/how-quickly-does-dexamethasone-work/
- Verywell Health. (2023, Sept 10). Corticosteroid-induced osteoporosis. https://www.verywellhealth.com/what-to-know-about-corticosteroid-induced-osteoporosis-190176
- Horne, R., & Cooper, D. (2017). Rifampin drug interactions. JAMA Internal Medicine, 177, 452-453. https://doi.org/10.1001/jamainternmed.2016.8900
- PediatricEducation.org. (2021, June 7). Indications for using dexamethasone. https://pediatriceducation.org/2021/06/07/what-are-some-indications-for-using-dexamethasone/
- Xiao, J., et al. (2023). Optimal approach to contrast-media extravasation. Cleveland Clinic Journal of Medicine, 90, 292-298. https://doi.org/10.3949/ccjm.90a.2022
How soon does relief begin after a 4 mg IV dose?
Non-genomic actions can alleviate symptoms within 10 minutes IV and about one hour IM.[33]
Is dexamethasone useful for viral croup in adults?
Case series support a single 0.6 mg/kg IM dose for moderate airway edema when epiglottitis is excluded.[34]
What is a steroid “flare”?
Up to 10 % of tendon-sheath or joint injections cause transient pain and erythema peaking at 24-48 h, resolving without intervention.[35]
Does a perioperative 8 mg dose raise infection risk?
Large, randomized trials show no significant increase in surgical-site infections with a single intra-operative dose.[36]
What if dexamethasone extravasates?
Localized burning and itching usually subside with elevation and warm compresses; tissue necrosis is exceedingly rare.[37]
How long does dexamethasone stay active?
Biological effects can persist 72 h owing to a prolonged half-life and sustained receptor occupancy.[38]
Will one injection weaken bones?
Single or brief courses do not measurably reduce bone mineral density; risk emerges after weeks-to-months-long exposure.[39]
Is grapefruit juice a concern?
Grapefruit modestly inhibits intestinal CYP3A4 and could slightly raise levels, but clinically significant effects are uncommon with occasional intake.[40]
Is it safe in infants under two?
Weight-based dosing is accepted for bronchiolitis and adrenal crises in infants, with monitoring for hyperglycemia.[41]
Does it interact with IV contrast dye?
No pharmacokinetic interaction exists; corticosteroids are instead used prophylactically to prevent contrast reactions.[42]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
Administration Instructions
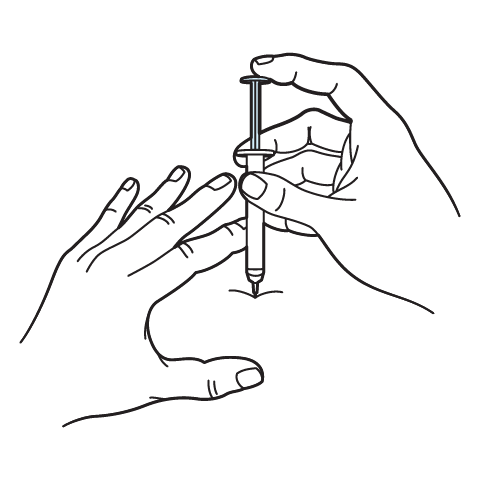
Intramuscular Injection Instructions
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Ketorolac Tromethamine Injection
Ketorolac Tromethamine Injection Prednisone Tablets
Prednisone Tablets Hydrocortisone Tablets
Hydrocortisone Tablets Diphenhydramine Injection
Diphenhydramine Injection Ondansetron Injection
Ondansetron Injection Ascorbic Acid (Vitamin C) Injection
Ascorbic Acid (Vitamin C) Injection Vitamin B-Complex Injection
Vitamin B-Complex Injection Glutathione Injection
Glutathione Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo) Cyanocobalamin (Vitamin B12) Injection
Cyanocobalamin (Vitamin B12) Injection