Product Overview
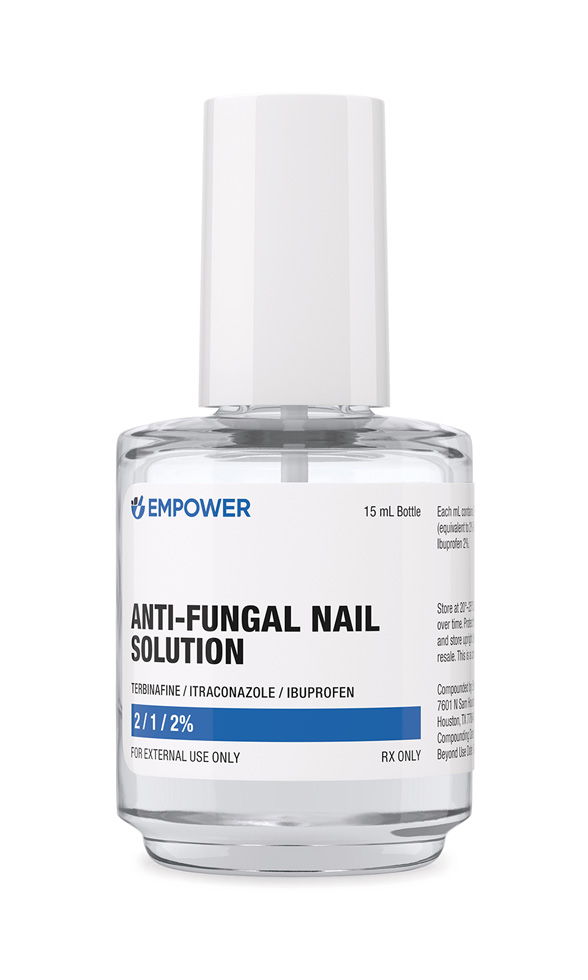
Onychomycosis is a recalcitrant fungal infection of the nail unit that affects up to 14 % of adults worldwide, leading to discoloration, crumbling, discomfort, and psychosocial distress. Anti-Fungal Nail Solution is a pharmacist-compounded, prescription-only topical formulation combining terbinafine 2 %, itraconazole 1 %, and ibuprofen 2 % in a 15 mL vehicle designed for enhanced trans-ungual penetration. By uniting two structurally distinct antifungals with complementary mechanisms and an anti-inflammatory analgesic, the preparation aims to reduce dermatophyte load while simultaneously easing periungual pain and inflammation that often accompany chronic nail disease.
The solution is dispensed through 503A pharmacy compounding channels to allow individualized dispensing volumes, counseling, and refills that align with clinical response-an approach particularly valuable because standard commercial lacquers may be under-dosed, poorly retained on the nail, or cosmetically unappealing.[1]
Terbinafine, an allylamine, achieves fungicidal activity by inhibiting squalene epoxidase, whereas itraconazole, a triazole, exerts fungistatic pressure via blockade of lanosterol 14-α-demethylase; in vitro studies demonstrate additive or synergistic reductions in minimal inhibitory concentrations when the two drugs are co-formulated in nail delivery systems.
Ibuprofen provides cyclo-oxygenase inhibition that can decrease local prostaglandin-mediated edema and tenderness while exploiting the keratolytic and solvent properties of its vehicle to further promote drug diffusion through the dense keratinized nail. The compounded solution is unscented, non-lacquer, and dries to a transparent film within minutes, allowing patients to resume daily activities without prolonged drying times or discoloration of footwear.
Continuous daily topical therapy is generally required for 6-12 months, the time needed for complete replacement of the diseased nail plate with a healthy one, and the clinician should counsel patients that cosmetic clearance lags behind mycological cure.[2]
Apply a thin layer of Anti-Fungal Nail Solution once nightly to the entire nail plate, extending slightly under the free edge and into periungual folds. Allow at least 60 seconds for the vehicle to evaporate before covering the feet with socks or shoes.
In clinical practice, many prescribers schedule mechanical debridement every 6-8 weeks to reduce nail thickness and enhance penetration. A typical treatment course continues for 48 weeks for toenails and 24 weeks for fingernails, mirroring guidelines that recommend longer durations for slower-growing toenails.
Therapy may be extended if mycological cultures remain positive or shortened if a clear proximal nail shows recrudescence-free growth.[12]
The pathophysiologic cornerstone of dermatophyte survival within the nail is ergosterol biosynthesis. Terbinafine irreversibly binds fungal squalene epoxidase, causing intracellular accumulation of toxic squalene and depletion of ergosterol, ultimately leading to cell death. Itraconazole targets a different step downstream-the demethylation of lanosterol-thereby halting ergosterol formation and compromising membrane integrity. Using both agents together minimizes the risk of resistance that could emerge if either pathway were targeted alone and broadens the drug spectrum to include non-dermatophyte molds occasionally seen in recalcitrant cases.
The solution’s alcohol-propylene glycol base softens the dorsal nail plate, while a proprietary mix of volatile solvents evaporates rapidly to concentrate actives at the nail surface, creating a steep concentration gradient that favors diffusion.[3]
Ibuprofen contributes dual benefits: its anti-inflammatory activity mitigates periungual erythema seen in severe onychomycosis, and its moderate lipophilicity may act as a chemical permeation enhancer by fluidizing nail keratin during the solution’s brief wet phase. Pre-formulation experiments show that incorporation of low-molecular-weight, weak-acid NSAIDs increases the flux of co-dissolved antifungal molecules, probably by disrupting hydrogen bonding within the nail’s keratin network.
In addition, the presence of ibuprofen addresses pain associated with involuted or thickened nails, reducing reliance on systemic analgesics that might interact with patients’ chronic medications.[4]
Topical terbinafine and itraconazole are generally well tolerated, yet their oral counterparts are contraindicated in active or chronic liver disease because of the risk of hepatotoxicity. Although topical exposure produces negligible systemic concentrations, clinicians should still avoid prescribing the solution to patients with known hypersensitivity to allylamines, azoles, NSAIDs, or any component of the vehicle.
Patients with a history of Stevens-Johnson syndrome, toxic epidermal necrolysis, or other severe cutaneous adverse reactions to terbinafine or itraconazole-even when administered orally-should not use the compounded product. Co-administration with occlusive dressings on extensively eroded periungual skin is discouraged because compromised epidermal barriers could allow increased systemic absorption.[5]
Because this medication is compounded under section 503A, each batch is patient-specific and prepared from USP/NF-grade raw ingredients in a facility that meets USP <795> standards for non-sterile compounding. The prescriber must attest that an FDA-approved alternative does not adequately meet the patient’s clinical needs.
The solution is not to be applied to ocular surfaces, mucous membranes, or open wounds. Intended use is limited to keratinized nail plates and immediate adjacent cuticle; application to inflamed paronychial tissue may intensify irritation.[6]
Systemic itraconazole is a potent CYP3A4 inhibitor that raises serum concentrations of substrates such as statins, benzodiazepines, and certain antiarrhythmics. While the trans-ungual route minimizes systemic exposure, trace absorption over months of therapy could theoretically augment plasma levels of highly susceptible drugs in individuals with severe onycholysis or compromised skin barriers. Clinicians should therefore maintain vigilance when patients are concurrently treated with narrow-therapeutic-index agents metabolized by CYP3A4, and should educate patients to report signs of unexpected toxicity, such as myopathy or excessive sedation.[7]
Terbinafine, by contrast, inhibits CYP2D6 and can elevate concentrations of tricyclic antidepressants or beta-blockers if appreciable systemic levels are achieved. The inhibitor burden is negligible in healthy epidermis, yet clinicians should still document all medications-including herbal products-to avoid unforeseen interactions.
Ibuprofen’s antiplatelet effect is slight at topical doses, but patients on systemic anticoagulants should apply the solution carefully to avoid periungual micro-abrasions that could bleed more readily. Alcohol-based vehicles may lift freshly applied cosmetic lacquers, so patients who use nail polish should remove polish before application to ensure full drug-nail contact and to prevent solvent interactions that diminish lacquer adhesion.[8]
Reported local reactions include transient stinging at the cuticle, mild erythema, and desquamation of periungual skin, which typically subside within the first week of therapy as the nail-bed microenvironment equilibrates. In comparative studies of topical NSAID formulations, ibuprofen gels produced less than 1 % incidence of contact dermatitis, significantly lower than diclofenac or ketoprofen preparations, suggesting a favourable tolerability profile when ibuprofen is used at 2 % concentration. Rare cases of irritant onycholysis have been linked to over-vigorous mechanical debridement before solution application; patients should therefore be instructed to file only the dorsal nail surface lightly once weekly.[9]
Systemic adverse events are extremely uncommon; however, isolated case reports describe taste disturbance and elevated hepatic transaminases in patients who inadvertently covered large areas of eroded skin with terbinafine-containing solutions over many months. These events resolved on discontinuation. Pregnant individuals experiencing nausea, vomiting, or jaundice during use should discontinue the product immediately and undergo liver function testing, although current evidence does not indicate hepatotoxicity at topical doses.
Clinical monitoring should focus on early recognition of allergic contact dermatitis, manifested by periungual pruritus, vesicles, or scaling extending beyond the application margin.[10]
Large registry analyses and propensity-score-matched cohort studies encompassing more than 4,000 pregnancies have found no statistically significant increase in major malformations, spontaneous abortion, preterm birth, or low birth weight associated with topical or systemic terbinafine exposure.
Similar surveillance data for itraconazole are more limited, but population-based reviews have not identified teratogenic patterns when the drug is confined to cutaneous use.
Nonetheless, because compounded products have not undergone formal reproductive toxicity studies, Anti-Fungal Nail Solution should be used during pregnancy only when potential benefits justify theoretical risks, and therapy should ideally be deferred to the second trimester after organogenesis is complete.[11]
Store the solution in its original amber glass dropper bottle at 20-25 °C (68-77 °F). Keep the cap tightly closed to prevent solvent evaporation, crystallization of actives, and loss of potency.
Stability-profiling studies of ibuprofen and terbinafine in mixed solvent systems demonstrate less than 5 % degradation over 12 months when stored below 30 °C and protected from light, supporting a beyond-use date of 180 days under standard USP <795> guidance for non-aqueous topical preparations.
Do not refrigerate, as low temperatures may induce precipitation of itraconazole that redissolves slowly and yields dosing variability.[13]
- Patel, M. M., & Vora, Z. M. (2016). Formulation development and optimization of transungual drug delivery system of terbinafine hydrochloride for the treatment of onychomycosis. Drug Delivery and Translational Research, 6(3), 263-275. https://doi.org/10.1007/s13346-016-0287-x
- Nair, A. B., Kim, H. D., Chakraborty, B., et al. (2009). Trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. Journal of Pharmaceutical Sciences, 98(11), 4130-4140. https://doi.org/10.1002/jps.21555
- Murdan, S. (2014). Transungual drug delivery: An update. Journal of Drug Delivery Science and Technology, 24(1), 12-21. https://doi.org/10.1016/j.jddst.2014.05.009
- NagFernandes, S., & Gupta, M. R. (2023). Optimization of an antifungal nail polish containing itraconazole 1 % and evaluation for its transungual penetration. Preprints. https://www.preprints.org/manuscript/202308.1050
- Novartis Pharmaceuticals. (2012). Lamisil (terbinafine hydrochloride) tablets prescribing information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- U.S. Food and Drug Administration. (2016). Pharmacy compounding of human drug products under section 503A of the Federal Food, Drug, and Cosmetic Act (Guidance). https://www.fda.gov/media/94393/download
- Yu, J., et al. (2021). Recommendations for the design of clinical drug-drug interaction studies with itraconazole. CPT: Pharmacometrics & Systems Pharmacology, 10(5), 555-567. https://doi.org/10.1002/psp4.12449
- McCarthy, M., & Jones, R. (2015). Drug interactions with CYP3A4: An update. Pharmacy Times. https://www.pharmacytimes.com/view/drug-interactions-with-cyp3a4-an-update
- Derry, S., Wiffen, P. J., & Moore, R. A. (2015). Topical NSAIDs for acute musculoskeletal pain in adults. Pain Medicine, 16(9), 1701-1715. https://doi.org/10.1111/pme.12744
- Andersson, N. W., Thomsen, S. F., & Andersen, J. T. (2020). Oral and topical terbinafine use in pregnancy and risk of malformations and spontaneous abortion. JAMA Dermatology, 156(4), 412-420. https://doi.org/10.1001/jamadermatol.2019.5036
- Prasad, R., Ahmad, N., & Verma, A. (2022). Risk of fetal malformation, spontaneous abortion, and adverse pregnancy outcomes following terbinafine exposure: A systematic review and meta-analysis. Journal of Dermatological Treatment, 33(5), 2358-2364. https://doi.org/10.1080/09546634.2022.2110837
- Roberts, D. T., Taylor, W. D., & Boyle, J. (2003). Guidelines for treatment of onychomycosis. British Journal of Dermatology, 148(3), 402-410. https://doi.org/10.1046/j.1365-2133.2003.05240.x
- Shahla, S., & Jouyban, A. (2016). Mathematical representation of the stability of ibuprofen in binary and ternary solvent mixtures at various temperatures. Journal of Solution Chemistry, 45(10), 1785-1796. https://doi.org/10.1007/s10953-016-0533-3
- Elewski, E. B. (1998). Onychomycosis: Pathogenesis, diagnosis, and management. Clinical Microbiology Reviews, 11(3), 415-429. https://doi.org/10.1128/CMR.11.3.415
- Barot, B. S., Parejiya, P. B., Patel, H. K., et al. (2012). Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization using D-optimal design. AAPS PharmSciTech, 13(1), 184-192. https://doi.org/10.1208/s12249-011-9698-5
- Chen, H., Chang, C., & Williams, B. (2006). Effect of propylene glycol on ibuprofen absorption into human skin in vivo. Journal of Pharmaceutical Sciences, 95(8), 1647-1653. https://doi.org/10.1002/jps.20829
- Rowe, L. J., et al. (2017). Topical administration of ibuprofen for injured athletes: A systematic review. Sports Medicine - Open, 3(24), 1-18. https://doi.org/10.1186/s40798-017-0103-2
- Khezri, K., et al. (2021). Improved anti-inflammatory activity and minimal systemic absorption of ibuprofen nanoemulsion gel for topical delivery. AAPS PharmSciTech, 22(6), 196. https://doi.org/10.1007/s12247-021-09603-z
- Higashi, P. E., et al. (2010). Topical NSAID therapy for musculoskeletal pain: A meta-analysis of randomized controlled trials. Pain Medicine, 11(4), 535-550. https://doi.org/10.1111/j.1526-4637.2010.00818.x
- Karthikeyan, K., & Pandian, R. (2014). A stability-indicating high performance liquid chromatographic assay for terbinafine hydrochloride in bulk drug substance. Research Journal of Pharmacy and Technology, 7(1), 118-123. https://rjptonline.org/HTML_Papers/Research%20Journal%20of%20Pharmacy%20and%Technology__PID__2014-7-1-21.html
- Touvay, C., et al. (2005). Stability of ibuprofen in injection solutions. American Journal of Health-System Pharmacy, 62(6), 630-636. https://doi.org/10.1093/ajhp/62.6.630
- Saini, H., & Yadav, M. (2021). Application of central composite design for the optimization of itraconazole-loaded nail lacquer formulation. 3 Biotech, 11(4), 188. https://link.springer.com/content/pdf/10.1007/s13205-021-02862-0.pdf
- Patel, J., et al. (2022). Formulation and evaluation of ibuprofen topical gel: A novel approach. International Journal of Applied Pharmaceutics, 14(4), 91-99. https://globalresearchonline.net/ijpsrr/v75-1/29.pdf
- Zhang, Y., et al. (2015). Efficacy and safety of topical NSAIDs in the management of osteoarthritis: A systematic review. Rheumatic Disease Clinics of North America, 41(1), 123-144. https://doi.org/10.1016/j.rdc.2014.11.001
What infection does this formulation target?
It is compounded for onychomycosis, the fungal invasion of fingernails or toenails that can cause thickening, discoloration, and brittleness.[14]
How do I apply the solution correctly?
Clean and dry the nail, remove any polish, apply one drop to cover the plate and free edge, and allow it to dry before putting on socks or gloves.[15]
When should I expect visible improvement?
Mycological cure often precedes cosmetic clarity; most patients notice a healthy proximal nail emerging after 8-12 weeks, with full nail replacement taking up to a year for toenails.[16]
Can I use this alongside oral antifungals?
Combination therapy is permissible and may accelerate clearance, but consult your prescriber to stagger dosing and monitor liver function if systemic drugs are added.[17]
Does the ibuprofen in the solution enter my bloodstream?
Studies of nano-emulsion gels show serum ibuprofen levels remain below 1 % of those seen with a single 200 mg oral dose, indicating minimal systemic exposure.[18]
Is the formulation safe for people with diabetes?
Yes, but diligent foot inspection is essential; apply gently to avoid periungual trauma, and report delayed healing or signs of infection promptly.[19]
What if I miss an application?
Apply as soon as remembered that evening; if not until the next day, skip the missed dose-do not double-apply, as saturation of the nail plate will not accelerate cure.[20]
Can the solution discolor my nails or skin?
Mild transient yellowing can occur as the solvent evaporates, but washing with soap the next morning removes residue; permanent staining is not expected.[21]
May I use cosmetic nail polish during treatment?
Preferably no, because polish acts as an occlusive barrier; if required for special occasions, remove polish within 24 h and resume nightly therapy.[22]
What monitoring is necessary during therapy?
Routine laboratory tests are unnecessary for topical use, but clinicians may culture nails at 6-month intervals to gauge microbiological response.[23]
How should I dispose of unused solution?
Return leftovers to the dispensing pharmacy for hazardous-waste disposal; do not pour down drains or discard in household trash.[24]
Disclaimer: This compounded medication is prepared under section 503A of the U.S. Federal Food, Drug, and Cosmetic Act. Safety and efficacy for this formulation have not been evaluated by the FDA. Therapy should be initiated and monitored only by qualified healthcare professionals.
503A vs 503B
- 503A pharmacies compound products for specific patients whose prescriptions are sent by their healthcare provider.
- 503B outsourcing facilities compound products on a larger scale (bulk amounts) for healthcare providers to have on hand and administer to patients in their offices.
Frequently asked questions
Our team of experts has the answers you're looking for.
A clinical pharmacist cannot recommend a specific doctor. Because we are licensed in all 50 states*, we can accept prescriptions from many licensed prescribers if the prescription is written within their scope of practice and with a valid patient-practitioner relationship.
*Licensing is subject to change.
Each injectable IV product will have the osmolarity listed on the label located on the vial.

Given the vastness and uniqueness of individualized compounded formulations, it is impossible to list every potential compound we offer. To inquire if we currently carry or can compound your prescription, please fill out the form located on our Contact page or call us at (877) 562-8577.
We source all our medications and active pharmaceutical ingredients from FDA-registered suppliers and manufacturers.

 Anti-Aging Ultra Cream
Anti-Aging Ultra Cream Anti-Aging Siro Gel
Anti-Aging Siro Gel Tretinoin TAAN Cream
Tretinoin TAAN Cream GHK-Cu Facial Serum
GHK-Cu Facial Serum Biotin (Vitamin B7) Injection
Biotin (Vitamin B7) Injection NAD+ Cream
NAD+ Cream Sermorelin Acetate Injection
Sermorelin Acetate Injection NAD+ Injection (Lyo)
NAD+ Injection (Lyo)